Researchers' Zone:
Are ‘DARPins’ the new heroes of medicine?
From cancer to COVID-19, DARPins are the game-changers that could transform our battle against diseases
Proteins play a vital role in biological processes. They are involved in a wide variety of functions, including digesting the food we eat, replicating DNA during cell division, and transporting molecules within our cells.
Antibodies, in particular, are important proteins that help the immune system fight off diseases. However, traditional antibodies have limitations in terms of their size and stability.
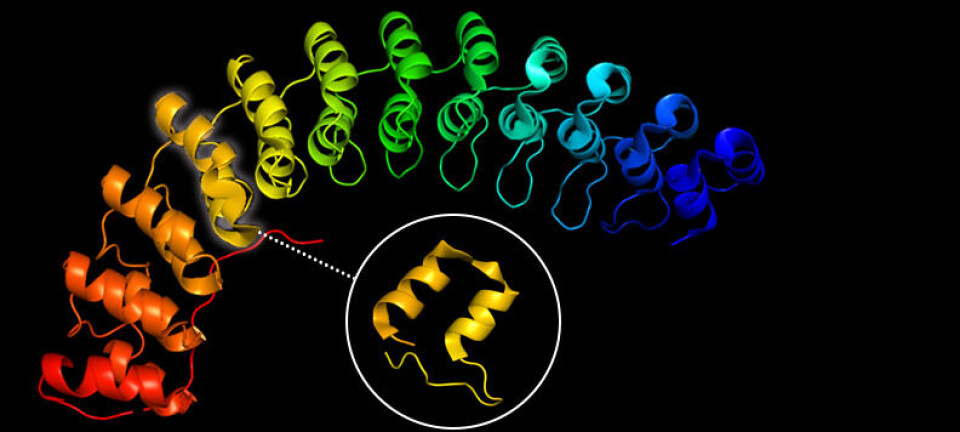
This is where DARPins come in. DARPins, or designed ankyrin repeat proteins, are a unique type of protein scaffold that offer many advantages over traditional antibodies.
In this article, we'll dive into what DARPins actually are, how they were discovered and the huge potential of these tiny proteins.
What are DARPins?
While DARPins are similar to antibodies in their ability of highly specifically binding different types of targets; unlike antibodies, DARPins are small, stable, and cheaply produced in large quantities.
They also have a concavely shaped binding region, whereas antibodies have convex loops. This gives DARPins a better shot at binding small targets, with high specificity.
Their small size also permits greater tissue penetration, enabling them to access targets that are not reachable by antibodies.
The history of DARPins dates to the early 1990s, when researchers discovered that ankyrin repeat proteins could be used as a scaffold for protein engineering.
Ankyrins: the key to finding DARPins
Ankyrin repeats are incredibly abundant in nature and are present in a wide range of organisms, from single-celled bacteria to complex multicellular organisms like humans.
They are small proteins, having multiple repeats of the same sequence that are involved in protein-protein interactions that primarily maintain the structural integrity of cells and tissues by acting as molecular scaffolds.
Researchers quickly realized that these ankyrin repeats could be manipulated to create new proteins with specific binding properties.
Based on the research in ankyrins, the first DARPins were created in the late 1990s.
A team of researchers at the University of Zurich screened millions of different DARPin variants to find one that could bind to the human epidermal growth factor receptor 2 (HER2), a protein that is overexpressed in certain types of cancer.
DARPins against Covid-19
Since then, DARPins have been used in a variety of applications, including research, diagnostics, and therapeutics.
Indeed, DARPins have been used successfully in the treatment of several diseases, including cancer, macular degeneration, and COVID-19.
In pre-clinical studies, researchers have shown that DARPins can effectively bind to the spike protein of COVID-19, preventing the virus from entering human cells.
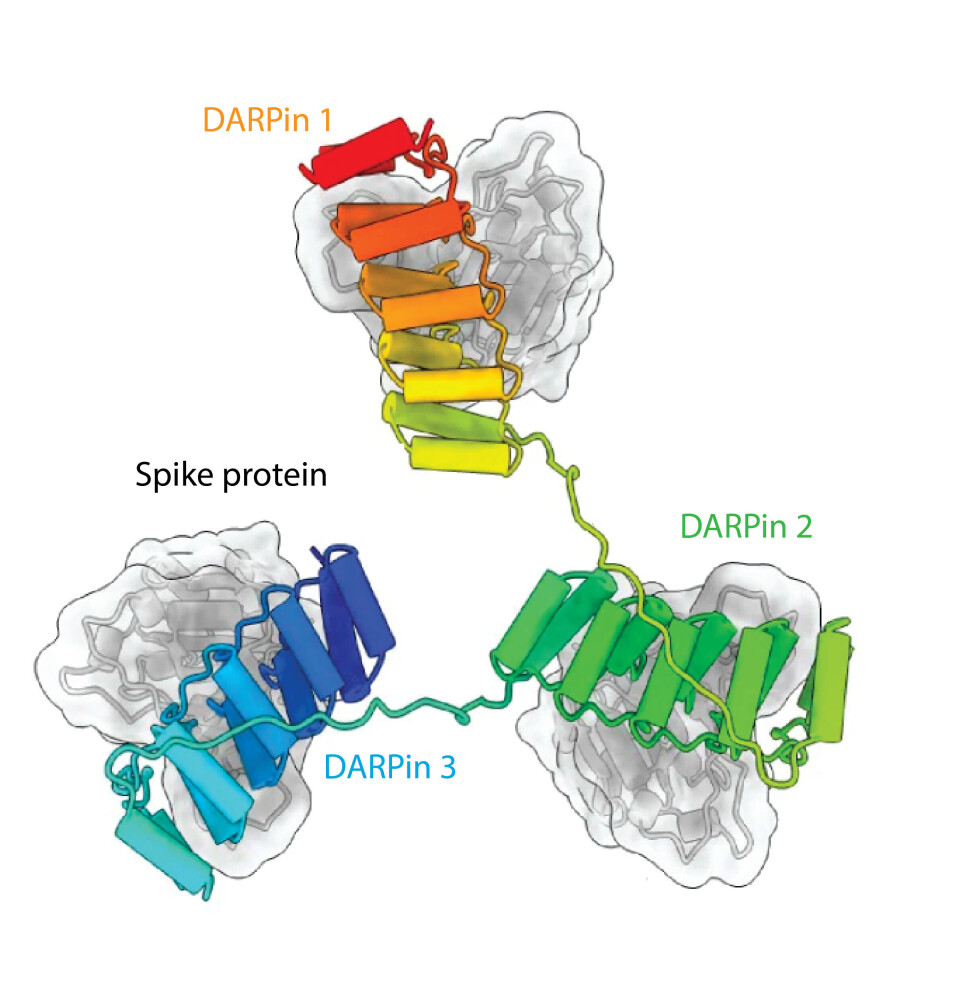
This led to the development of a vaccine which is now undergoing clinical trials. The vaccine candidate, ‘Envisobep’ can bind to all COVID-19 variants, including the highly contagious OMICRON variant.
It was engineered to have three portions, each binding to three different epitopes of the spike protein. This multivalent mode of binding results in a high protection rate.
An interesting observation from the Phase 2 trials of Envisobep was that no adverse effects such as cellular toxicity, or triggering of an immune response were observed even at high doses.
Used in cancer treatments
That Envisobep does not cause an immune response shows the low immunogenicity of DARPINs, a factor that could be attributed to the abundance of ankyrin proteins in the human body.
This is because our body’s defense mechanism makes it reactive to proteins that are of external origin, while tolerating proteins that are endogenous.
In the case of cancer, DARPins have been designed to specifically target tumor cells and deliver drugs directly to them, minimizing side effects and improving efficacy.
MP0250 is a multi-domain DARPin designed to target the hepatocyte growth factor (HGF) and the vascular endothelial growth factor (VEGF), which are signaling proteins that cause increased growth and invasion of cancer cells.
Upon binding to these proteins, MP0250 can help to stop the growth and spread of cancer cells by inhibiting the process of cancerous tissue formation.
In macular degeneration, DARPins have been developed as an alternative to traditional antibody-based therapies, offering greater stability.
This disease is a progressive eye condition that affects the macula, the central part of the retina responsible for sharp, central vision. It is a leading cause of vision loss and impairment, particularly among older adults.
Here, a drug called Abicipar made it to phase 3 clinical stage before being declined. However, the company with the rights to the drug, Molecular Partners AG, is expected to have resumed its development.
The big potential
The increasing application of DARPins in healthcare is driven by advances in rapid lab screening techniques to diagnose patients (?) and recombinant DNA technology that forms the basis for different kinds of treatments(?).
Screening methods involve exposing the DARPin library to the target protein in some kind of assay, to select the DARPin that binds the strongest.
Along with that, recombinant DNA technology enables scientists to cut and insert DNA to create modified genes; and also to take the genes for the desired product, and clone them in high producing, fast growing expression systems like bacteria or yeast.
In the near future, new computational screening techniques like RF Diffusion could also be applied to the development of DARPins as therapeutic candidates, since the rigidity of DARPins makes them easy to model.
This type of computational screening could assist in the rapid discovery of DARPin binders, thus saving time and speeding up the drug development pipeline.
We need to know more about the long-term effects
The cutthroat competition in the pharmaceutical industry and the conservativeness of its big players pose a challenge to the rapid implementation of DARPins.
While DARPins have shown promise in preclinical studies and early-phase clinical trials, there is limited data available regarding their long-term safety and efficacy.
Further clinical research and validation are needed to establish their clinical utility and address potential drawbacks. Furthermore, while their small size might aid them in penetrating tissue and being distributed quickly, they have relatively short half-lives in the bloodstream (only a few hours).
This might be sufficient for certain acute diseases but could pose challenges in the use of DARPins for chronic diseases. Here, frequent dosing or continuous infusion to maintain therapeutic levels might be required.
However, this problem can be solved in many ways, such as attaching other larger molecules to them or fusing several DARPins together as was done for Envisobep.
All in all, with continued research and development, DARPins have the potential to play a major role in the treatment of future diseases and present an exciting new tool in our therapeutic toolbox.