New key to better drugs
Researchers have uncovered an important mechanism that will result in more effective drugs.
The food, perfume and drugs industries often develop and produce new taste additives, scents and drugs through trial and error in a laboratory until they find a formula that works. It’s something they are really good at.
But so far no-one has really understood the mechanism behind the process – what actually makes some molecules react appropriately for the task in hand.
In other words, the developers have produced something they knew would work without understanding why it works.
For the first time, the missing link has been pinpointed by a group of researchers at Aarhus University working together with Canadian researchers. Their work has just been published in the prestigious journal Science.
Helps us understand chiral connections
“Our results can be important for how we develop taste additives, scents and drugs in the future, as the results will help us work more systematically rather than groping our way blindly,” says Professor Bjørk Hammer, who works at Aarhus University’s Interdisciplinary Nanoscience Center (iNANO) and at the university’s Department of Physics and Astronomy.
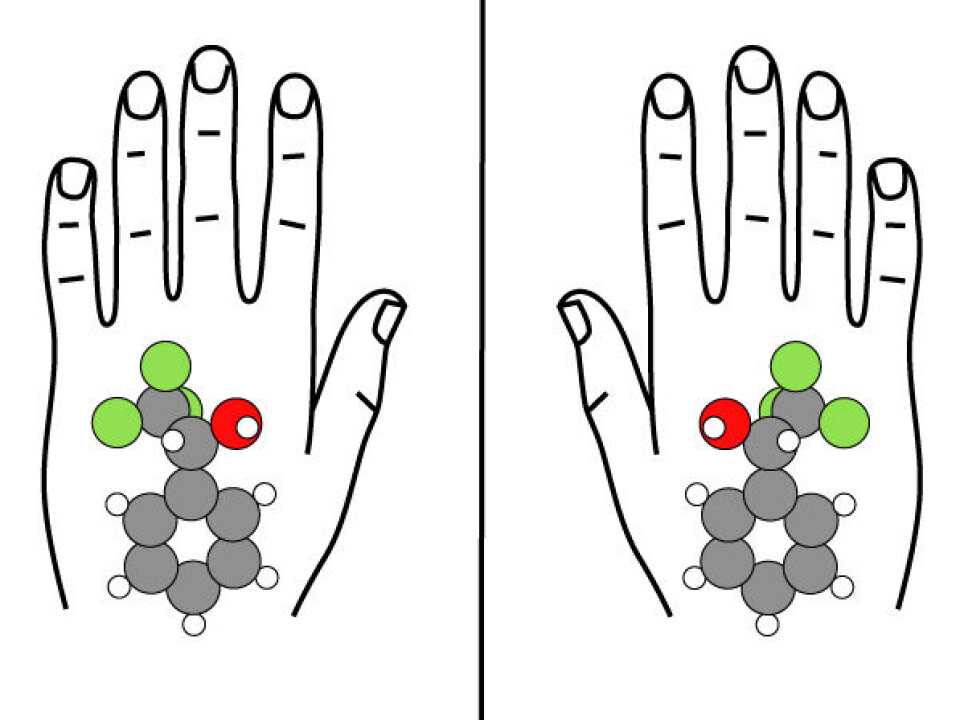
Hammer and his colleagues have discovered what a molecule looks like before and during a reaction. With this knowledge, the researchers realised they could see the mechanism behind chiral connections – phenomena in which a molecule starts off being symmetric but becomes asymmetric after a reaction (see factbox).
The concept of chirality means two things are mirror images of each other – like hands – and not identical. This applies to many chemicals, says the researcher.
“It’s like saying that the body is only made up of left-handed molecules that are mirrored – not right-handed molecules,” says Hammer. “We don’t know why. It’s a breach of symmetry that we don't understand. That’s why all living organisms are made up of only one type of chiral molecule.”
Towards better drugs
This means that drugs must be made of molecules that match the chiral structure of the body if they are to be effective.
In other words: a drug must be like a left-handed glove if left-handed molecules are to benefit from it. If the drug is a right-handed glove, it will not fit left-handed molecules and will therefore not work. The researcher says this situation can actually be dangerous for the patient and can have undesirable side effects.
It is therefore vital that the drugs industry can produce the correct chiral form of molecules. The food and perfume industries must also produce the correct chiral form of their molecules to isolate a certain taste or scent.
To date, that task has not been easy, as right-handed and left-handed chiral molecules share the same chemical formula and in many cases actually behave in the same way, says Hammer.
But the new discovery has enabled researchers to see what it is that results in left-handed and right-handed molecules. This knowledge makes it easier to control the chemical production processes, thereby achieving easier access to drugs with the correct chirality.
Read the article in Danish at videnskab.dk
Translated by: Michael de Laine











