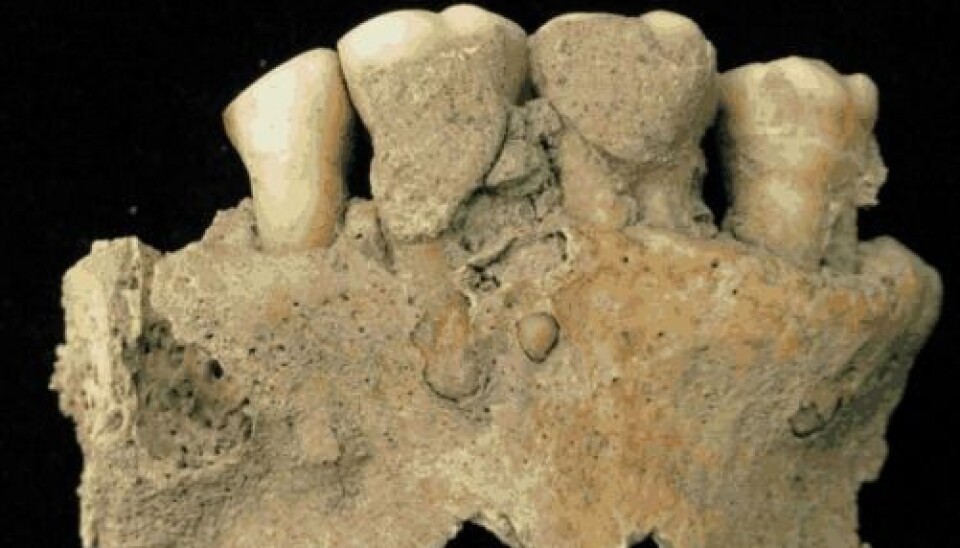
Tartar from ancient monks reveals serious diseases
Tartar in the mouths of 1,000-year-old monk skulls are a storehouse of information about past, and possibly also present-day, diseases. Analyses of the tartar indicate that humans were resistant to antibiotics 1,000 years ago.
Fossil DNA and proteins generally help us understand how lifestyle, diet and microbes have shaped our health. Now scientists have found a new source of information about the past: tartar in the teeth of ancient skulls.
With DNA and proteins hidden in the brown deposits, researchers can now see a detailed picture of what was going on in the mouths of German monks in the Middle Ages more than 800 years ago.
The molecules reveal a biological drama frozen in time – the immune system in the process of fighting bacterial attacks.
”This is like finding the archaeological remains of a prehistoric battle, only here it is at the molecular level,” says Enrico Cappellini, of the Center for Geogenetics at the University of Copenhagen, who headed the study.
Health problems may be linked to dietary changes
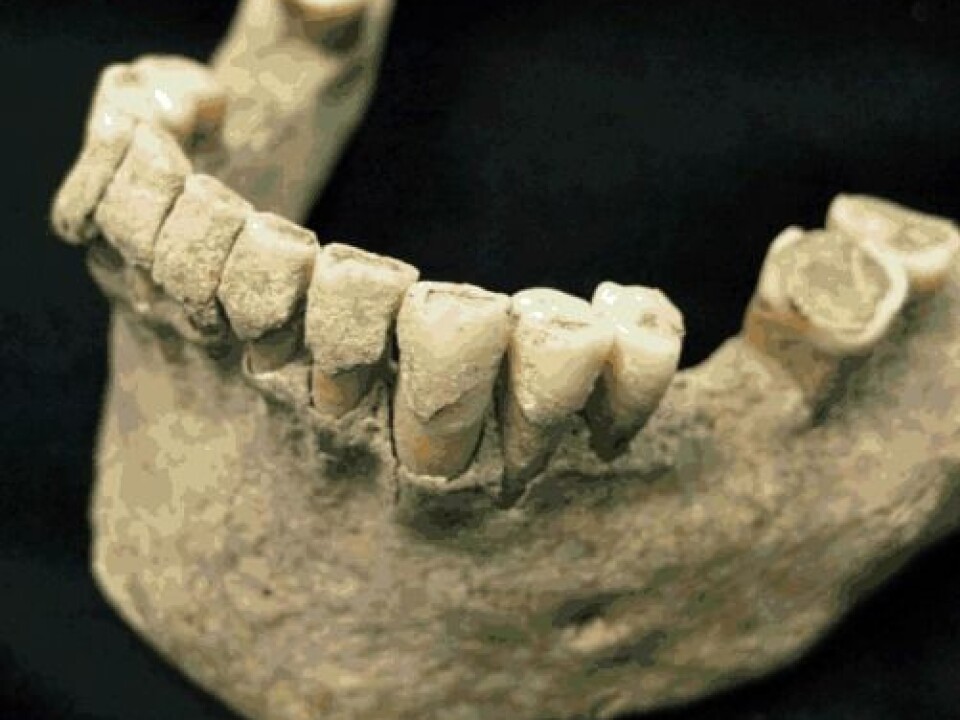
The study opens up great prospects. Scientists have in recent years shown that bacteria play an enormously important role in our health, including obesity, cardiovascular disease and diabetes.
Bacteria are not merely parasitic intruders; through evolution they have become an integral part of us – so much so that scientists refer to them as an extra organ: the ‘microbiome’.
”One of the big questions about the modern human microbiome is whether it has changed with lifestyle or whether we always had it,” says Professor Tom Gilbert, of the Center for Geogenetics, who also took part in the study.
You could, for instance, imagine that many of today’s health problems are associated with a microbiome, which is stretched to the breaking point, so to speak, as a result of our dramatic changes in diet and lifestyle over the centuries.
Tartar may solve the mystery

These kinds of answers may lie hidden in the tartar in our ancestors’ teeth.
This is a pioneering study in which Cappellini, together with an international team of scientists, has studied tartar from the teeth of four monks, who lived in the Middle Ages (circa 950-1200) and who were buried at the monastery in Dalheim, Germany.
Tartar is dense mineral layers made up of bacteria on the teeth surface, and it turns out that tartar is almost like a time capsule that preserves the biomolecules in a protective armour.
DNA studies reveal wide variety of bacteria
Using modern DNA technology and mass spectrometry, the researchers managed to identify large amounts of DNA molecules and proteins.
These analyses have revealed a wide selection of bacteria, including 40 that are known to play a role in various oral and dental diseases. They found:
- The S. mutans bacterium, which can lead to tooth decay.
- Several bacteria that are known to cause a risk of cardiovascular disease (A. actinomycetemcomitans, S. mutans and S. mitis)
- Three particularly common bacteria associated with inflammation of the gum tissue (T. forsythia, P. gingivalis and T. denticola).
They also found the proteins which show that the bacilli are heavily armed with surface proteins, which can wedge themselves in between our cells, and others that can help evade the immune system.
Genetic material from pathogenic bacteria also analysed
They also found human proteins – 43, of which 25 are important components of the immune system, including some that can prick holes in the bacteria’s outer shield and others that disarm the bacteria’s attack proteins.
This is the first time we see fossil samples from humans with bacteria that have antibiotic resistance – long before we started producing antibiotics industrially.
In other words, these are the weapons from a disease battle that was raging when the monks left. A battlefield that the scientists can pinpoint to the surface of the teeth, because the profiles in the jaw bone is highly different from that in the tooth.
The team found a lot of other interesting things. They also sequenced the genetic material of one of the disease bacteria, T. Forsythia, and found that it has several antibiotic resistance genes.
”This is the first time we see fossil samples from humans with bacteria that have antibiotic resistance – long before we started producing antibiotics industrially,” says Cappellini.
Monks ate pigs
This find indicates that the microbes in the mouth are an important source and a potential reservoir of antibiotic resistance genes, which can be shared among many different bacteria. The find may also prove to be important for our understanding of how problems with resistant bacteria arise today.
The researchers also found DNA from the food that the monks ate, and can see that remains of pork, cabbage and wheat were stuck between the teeth.
This all has the potential to be very interesting as we currently have a rather limited understanding of what our ancestors ate. This picture will become a lot clearer once the researchers have analysed the DNA fragments hidden in the tartar.
”It could be incredibly interesting to examine tartar from Neanderthals and humans who lived far back in time, 50,000 years ago or more,” says Cappellini.
An imbalance may have great effect on public health
It will be possible to study whether the great dietary changes associated with the transition from hunter-gatherers to agriculture have changed our bacterial flora and whether this affects our health.
”About 80 percent of the population experiences problems with diseases of the mouth over a lifetime, and that could suggest that our relationship with the microbes in our mouths has yet to be optimised to be symbiotic.”
We only have one cell for every ten bacterial cells that live on and inside our bodies, and it is remarkable that the bacteria in most parts of the body live in harmony with our own cells, but in our mouths they trigger regular visits to the dentist.
This may suggest a special imbalance, which could potentially have a major impact on public health – for example, we know that there is a correlation between the bacterial flora in the oral cavity and the risk of cardiovascular disease.
Proteins are more stable than DNA
The new study marks an important step into a new field of ’paleo-proteomics’, an area in which Cappellini and colleagues in 2012 conducted a landmark study of 43,000 year-old mammoth proteins.
In that study, they managed to sequence more than 100 different proteins and show that fossils may be the source of many more proteins than the little handful of the most abundant ones that the researchers have found so far.
The interesting thing is that proteins are more stable than DNA, which makes it potentially possible to study e.g. kinship further back in time than with DNA.
However, what is perhaps even more interesting is that the proteins open up an entirely different door to our prehistory, as the researchers can learn about the health condition of individuals based on the processes that took place then.
Proteins are very diverse
Whereas the DNA is identical in each one of the body’s cells, proteins are highly different e.g. from a liver cell to a nerve cell as an organism develops, and not least when disease sets in.
With the tartar from the monks, the researchers are now demonstrating how the proteins can detect a disease in full bloom, and how the organism responds to it.
This may be useful for many other studies in the future, for instance to determine whether an individual suffered from diseases such as diabetes, pneumonia or tuberculosis.
In combination with fossil DNA, the fossil proteins can become a powerful tool for studying how today’s widespread diseases such as obesity and diabetes have evolved through history.
Mutations may reveal increased diabetes risk
It is conceivable that mutations in fossil genetic material can tell us that an individual had an increased risk of diabetes, and that fossil proteins can then be used to identify the actual condition.
Now we could potentially get a unique snapshot of life in a lost world, and the ’paleo doctor’ will almost be able to take the ancient patient by the hand and make a diagnosis. And with more skeletons, we may even get a detailed picture of prehistoric public health and how it has developed.
-------------------
Read the Danish version of this article at videnskab.dk
Translated by: Dann Vinther