Scientists are removing CO2 from the air to make new products
Scientists are experimenting with a kind of artificial photosynthesis to replace oil in manufactured goods.
Nearly everything we use in daily life contains compounds made from oil: from computers to mobile phones, shoes, and tape. Now scientists are trying to make these substances directly from carbon dioxide (CO2).
Richard Heyn from SINTEF, Norway, leads a project called FUTUREFEED, which is designed to find ways to use CO2 in chemical processes as a replacement for oil.
In order to create new substances from CO2, the strong carbon bonds have to be weakened or broken completely.
“CO2 is such a stable molecule, we need a lot of energy to convert it," he says.
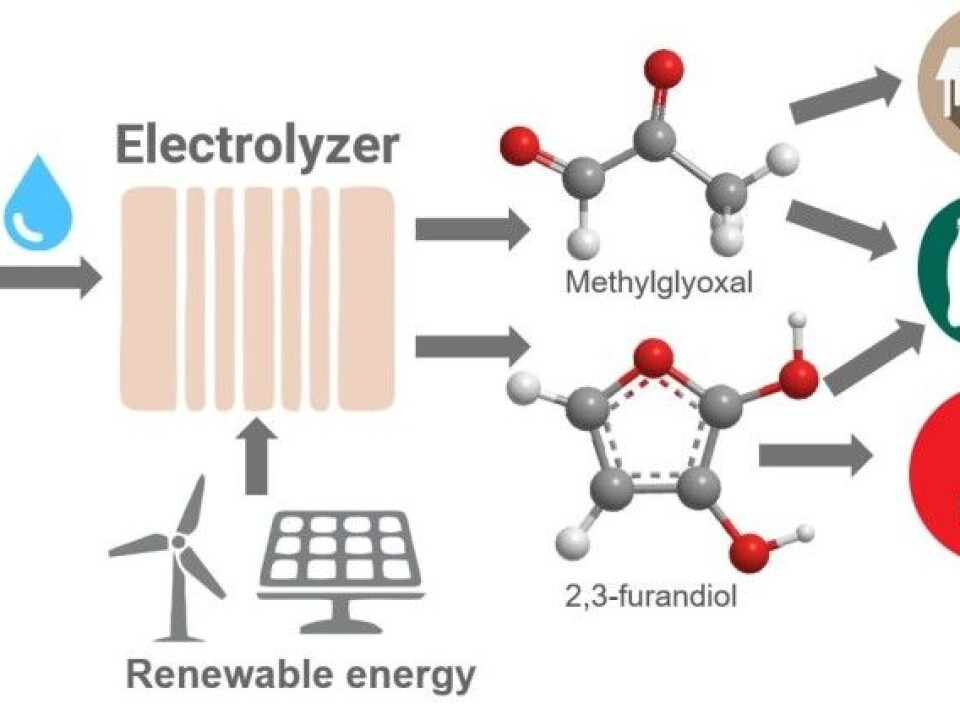
One way to do this is to add heat and pressure to a catalyst and other substances that the carbon atoms will react with. In theory, at least, this should allow the creation of a number of useful compounds. Heyn and his colleagues have tried using this process to make substances that are used in plexiglass and diapers.
However, the process as it is now requires far too much energy compared to the results they get. Heyn and colleagues have gone a step further by finding better catalysts that make the process more efficient, but the method is far from commercially feasible.
A new study at Rutgers University in the U.S., however, suggests a possible further step.
Read More: Pumping CO2 into volcanic rock transforms it into limestone in record time
Artificial photosynthesis
Instead of using heat and pressure to initiate reactions, the U.S. research group used electrolysis to convert CO2. The substance they use in the reaction is water, plain and simple. This is similar to artificial photosynthesis, in as much as it relies on energy, CO2, and water, to create new compounds.
The U.S. researchers have found new catalysts made of a mixture of phosphorus and nickel that are cheap and easily available. The catalysts help promote the reactions. They think their electrochemical process will allow them to create advanced molecules that can be transformed into plastic and a variety of other products.
Their results have now been published an article in Energy & Environmental Science.
"This work demonstrates that electrical energy is extremely efficient, fully 99 per cent, without the need for thermal energy. High temperature or high pressure is no longer necessary. It all happens at room temperature in a fuel-cell-like device called an electrolyzer,” writes Charles Dismukes, head of the new research, in an email to our sister site, Forskning.no.
In addition, the team is better able to control which substances are produced.
"This breakthrough can lead to the conversion of CO2 into valuable products and raw materials that can be used in the chemical and pharmaceutical industries," says Dismukes.
The researchers have patented the electrocatalyst and formed a start-up company called RenewCO2.
Read More: Danish scientists are turning CO2 into medicine
A golden future — hopefully
Heyn thinks the U.S. study is interesting, although he notes that the use of electrolysis to convert CO2 is not new. But the researchers from Rutgers have used a new material for the process, which is what makes it interesting, Heyn says.
Most of the processes used to transform CO2 rely on metals, often copper. The drawback of using copper as an electrode / catalyst is that the reactions are not that efficient and result in the production of many different products.
But the U.S. researchers had better control over the resulting products that were made.
Nevertheless, Heyn says, the technology is not yet ready to use. The new molecules were dissolved in water and they still need to find a way to separate the substances from the water. The process still needs to be faster and scaled up, he adds.
“But this is basic research, so we can’t expect them to solve all the problems right away,” Heyn says.
----------------
Read the Norwegian version of this article at forskning.no