Historic discovery: huge electric field occurs spontaneously in laughing gas
Scientists have discovered a new, astonishing electric phenomenon.
Danish scientists thought they had made a mistake when they discovered a huge electric field in a thin layer of solid nitrous oxide, commonly known as laughing gas.
As it transpires, however, they had discovered a new and astounding electrical phenomenon.
It was one of those ordinary days in the lab in the basement beneath Aarhus University. Two young physicists were examining how electrons pass through nitrous oxide.
They had frozen the gas to minus 233 °C making the molecules of the gas settle as an extremely thin film above a metal surface.
There wasn't supposed to be anything special about the ice-cold laughing gas, but the young researchers' measurements indicated something else - something completely different.
"They came upstairs and knocked on my door, saying ‘David, there's something not right’. At first we thought the experiment had gone wrong, because it wasn't supposed to be possible for a current to pass through the film and be detected. No external voltage was applied," remembers David Field, Professor at the Department of Physics and Astronomy at Aarhus University.
During the next few days, however, the scientists continued to see huge electric fields across the thin film of laughing gas, and it soon became clear that they had discovered an entirely new electrical phenomenon.
The findings have recently been published in Physical Chemistry Chemical Physics.
No one had discovered it before
Professor Field has dubbed the phenomenon ‘spontelectric'.
Since it’s first discovery in 2009, the scientists have discovered that the laughing gas is by no means the only material which shows the spontelectric phenomenon.
The electric field can reach more than 100 million volts per metre – i.e. enormous strength.
Professor Field and his colleagues have now examined more than 12 different materials and established that similar enormous electric fields occur spontaneously in common or garden materials such as carbon monoxide, propane, toluene and methyl formate.
All materials are frozen to extremely low temperatures in the form of a microscopically thin layer.
"In some cases, quite a few layers are necessary before the spontelectric effect manifests itself," says Field.
"When the experiment is conducted with toluene at 75 Kelvin, minus 198° C, more than a hundred layers are necessary for the effect to occur".
He believes that the spontelectric phenomenon happens because of a special interplay, in which the whole layer of molecules behave as much more than the sum of its parts, “in much the way as a beehive is much more than the sum of individual bees,” says Professor Field.
Researcher: an important discovery
One of the scientists who is researching 'spontelectrics' is Martin McCoustra from Heriot-Watt University in Scotland.
"Spontelectrics are an extremely important discovery. I think masses of exciting new theories will result from attempting to explain the phenomenon in the years to come," says McCoustra, head of the Department of Chemical Physics at Heriot-Watt University.
"This is the first time since the 1920s that anyone has discovered a new electrical form of solid material. The incredible thing is that work has been done on thin layers of materials -including laughing gas films - for more than half a century. Even so, no one has discovered this powerful electrical phenomenon" says Field.
The molecules are dipolar
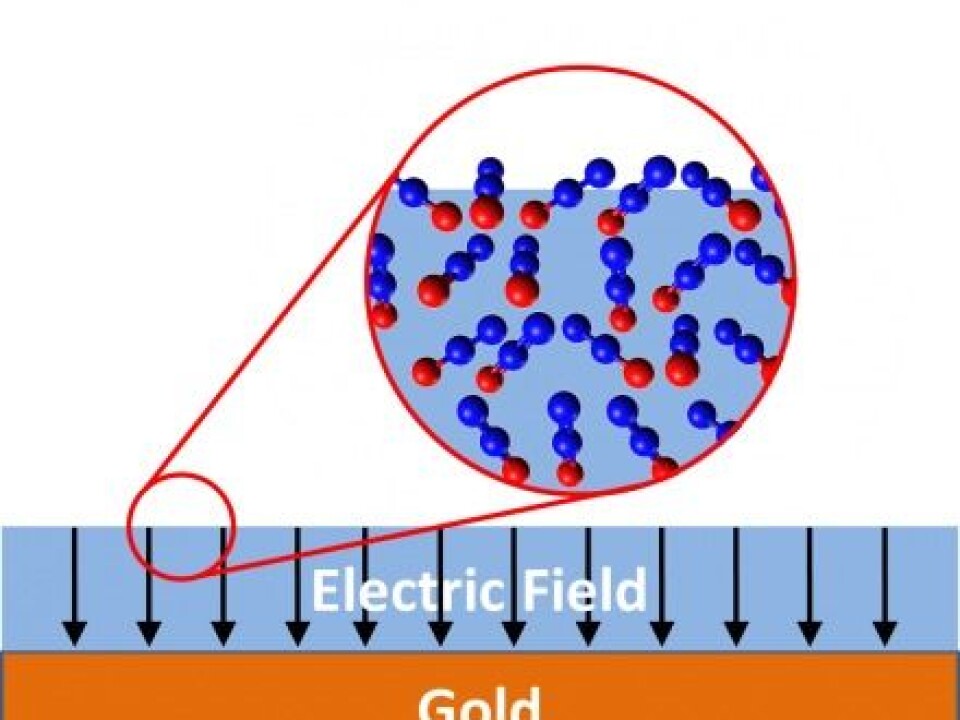
Laughing gas consists of dipolar molecules.
This means that one end of a laughing gas molecule has a slightly more negative electric charge than the other end.
The great majority of molecules are dipolar. For example, in laughing gas (N2O), the oxygen end of the molecule is negative compared with the positive nitrogen end.
When the spontelectric effect occurs, scientists have observed something unusual happening with the dipolar molecules:
Keeping to experiments conducted with a thin layer of laughing gas, the laughing gas molecules in the upper layer will arrange themselves in such a way that the positive end of the molecule pokes up towards the surface, creating a voltage on the surface of the film, Professor Field explains.
"Spontelectrics goes against our intuition. It doesn't seem to make any sense when you consider what we normally learn in physics and chemistry. The molecules arrange themselves entirely spontaneously - in a way that you would think they would not like. Fundamentally, we don't know what it is that makes them do it," says McCoustra.
Strange behaviour of molecules
The behaviour of dipolar molecules can be compared to magnets.
The opposite ends of the magnet will normally repel each other. In the same way, dipolar molecules attempt to position themselves in such a way that one molecule has its 'north pole' facing the 'south pole' of the other.
However, in experiments in which spontelectrics occur, the molecules do the exact opposite," says McCoustra.
“Under normal circumstances, the molecules will arrange themselves with the positive end towards the negative end. Instead, we see that they suddenly arrange themselves in unfavourable positions,”.
What can we use it for?
"Right now the discovery of the phenomenon is pure fundamental science, which contributes to our understanding of how solid substances behave," says Field.
Field is particularly interested in finding out whether the phenomenon plays a role when new stars are born.
Stars are created in huge clouds of dust and gas. In the middle of the clouds it is so cold that spontelectric carbon monoxide could potentially form. This would change the chemistry inside the cloud of dust and the way in which it would collapse to form a star.
"Spontelectrics may prove to have a big impact on our understanding of how stars are born," says Field.
"What can we use it for? To be frank, we don't know,” says McCoustra. “But that could have been said about many other scientific discoveries when they were made. It often takes a long time before we find out how to use a discovery”.
The spontelectric phenomenon was first seen in the ASTRID synchrotron laboratory (ISA) in the basement of Aarhus University.
----------------
Read the original story in Danish on Videnskab.dk
Translated by: Hugh Matthews