Nasal spray research was not legal – producers have to remove all information about study
The Norwegian company Viiral has violated several paragraphs in the Norwegian Health Research Act, according to the Norwegian Board of Health Supervision. Here are the reasons why their research was considered reckless and illegal.
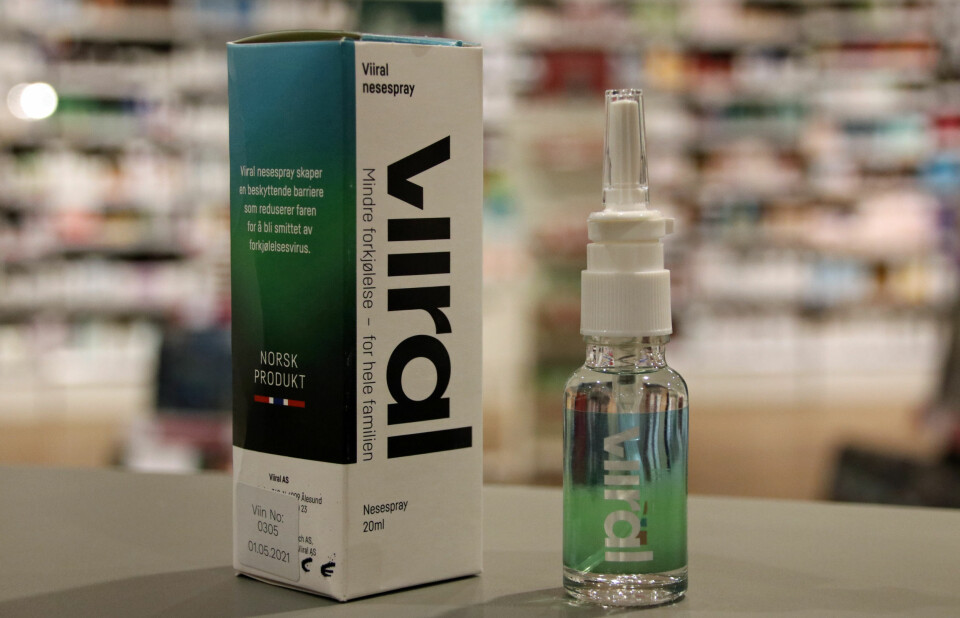
Viiral claims their nasal spray can help prevent colds.
In an interview with the Norwegian newspaper Dagens Næringsliv last year, the company referenced a 2017 study done on flight attendants working for SAS.
The active ingredient they were testing is called sialic acid, and is found in sea cucumbers.
According to their results, there were fewer days of sick leave among the group of employees using the medication than those who were given a placebo. However, the Norwegian Board of Health Supervision concluded that the nasal spray study was so deficient that it is indefensible. That means the results aren’t to be trusted.
The company has had to remove all information about the study, according to a report from the Norwegian Broadcasting Corporation (NRK).
“It isn’t often we see cases as deficient as this one,” says Toril Sagen, assistant director at the Norwegian Board of Health supervision. It isn’t often they handle cases regarding health science research, she adds.
A serious case
In Norway, all medical and health science research has to be pre-approved by one of four Regional Committees for Medical and Health Research Ethics (REK). This is to ensure that the research is done in a safe and responsible manner.
But Viiral didn’t get advance approval before starting its study.
The company did apply to REK, but the committee postponed their decision, asking for more information.
Viiral misunderstood the reply, thinking that it didn’t need to apply for approval after all.
“It’s really serious when they don’t even do the basic things, like applying for pre-approval. It’s the kind of thing you should know about when working in this field,” Sagen says.
The board said Viiral violated three paragraphs in the Norwegian Health Research act: §5 on responsibility, §6 on organization and §9 on pre-approval.
Irresponsible research
Unclear organization and no explanation for the choice of placebo are some of the mistakes the company made. “These failings may affect the scientific quality of the project. Research projects that aren’t conducted using proper scientific methods often aren’t defensible research,” the Board of Health Supervision write in their decision.
The board also found the information the company supplied to the participants to be inadequate. An important part of responsible research is supplying participants adequate information about what is involved in the study.
The fact that Viiral didn’t understand that they needed to apply for pre-approval of the project is a clear sign that they don’t have the experience to run a medical research project, according to the board. This is a clear breach of the paragraph on organization.

There’s no excuse
CEO Frode Fagermo realizes that they should have applied for the correct approval before starting the project.
“We made a mistake, and there’s no excuse for the way we handled the project,” Fagermo says.
Viiral applied for approval after the study was finished, but the application was rejected.
“We did the study with the best intentions, thinking we had done everything right. We operated in good faith,” Fagermo said.
He disagrees with the other legal violations the Board of Health supervision have pointed to.
“I don’t see what information was missing, and what could be considered indefensible.”
Cannot publicize the results
The board’s decision came in July, and Viiral was ordered to delete all personal information about participants, reports from the project, and any references to it.
“From our side, the case is closed. We’ve gotten confirmation that the information has been deleted. But if we hear anything about the results being published, we may have to take further action,” says Toril Sagen from the Norwegian Board of Health Supervision.
Collaborated with a university
The Karolinska Institutet in Stockholm was responsible for the research project, but was not involved in the application process, Fagermo says.
However, Dan Edwall, who has a doctorate from the Karolinska Institute and now works as a medical consultant in Viiral, was involved.
Hogne Vik, Viiral’s medical director, was also involved. He has previously studied the vitamin K2 and talked warmly about it as general manager of the company NattoPharma, which sells K2, according to an article in Norwegian national newspaper Aftenposten.
The Viiral nasal spray study was never published in a peer-reviewed academic journal, which would have involved a quality check from other researchers.
Nevertheless, Viiral told the press and its websites that the nasal spray had a documented effect.
Will study the product again
There was little risk of side effects or other disadvantages for the participants in the project, the Norwegian Board of Health wrote in its decision. The board has not assessed the actual results of the study.
Thus, whether the product actually prevents colds is unclear. This is the only user survey Viiral has conducted so far.
Viiral has applied to REK for pre-approval to start a new research project next year.
This time, the company has hired more help for writing its application.
“We have already taken steps to prevent us from falling into the same situation again. We see that we need to have more expertise,” Fagermo said.
“We want to do things right.”
Pharmacies stop selling the nasal spray
Atle Fretheim, research director at the Norwegian Institute of Public Health, went over the numbers from Viiral’s study last year. He looked at the amount of sick leave per participant and found no effect of the nasal spray, according to Aftenposten. He pointed out that a person with a high amount of sick leave can affect the outcome, if the study counts sick days regardless of who is sick.
This was rejected by Viiral in an article in Aftenposten. Medical Director Hogne Vik thought it was appropriate to count the total number of sick days among employees.
The nasal spray Viiral is for sale at a number of Norwegian pharmacies. The Norwegian Board of Health does not decide which products the pharmacies can choose to sell.
But several pharmacy chains have now pulled the product off the shelves, including the international chain Boots, according to NRK.
As of 20 November this year, another large Norwegian pharmacy chain, Apotek 1, also stopped the sale of Viiral nasal spray.
———
Read the Norwegian version of this article on forskning.no