Researchers' Zone:

Two young researchers: Ground-breaking discovery was a coincidence
Their research project went up in flames. But two young Danish researchers found something in the ashes that led to a surprising discovery about anticoagulants.
We started as PhD students at the University of Southern Denmark 8-9 years ago as young, hopeful and newly graduated pharmacists.
Tore was studying how the body distributes and metabolises drugs by conducting small clinical trials in healthy volunteers. Anton's project was more vaguely defined. Something with registry data. And something about investigating different aspects of treatment with the anticoagulant drug warfarin.

Warfarin has been used to treat patients for more than 50 years. Originally, it was discovered during an attempt to understand why cows died from eating a mouldy silage made from a sweet clover plant.
After many years of research, it became clear that the clover contained a substance similar to warfarin causing the cows to bleed to death. This knowledge was initially used to develop an effective rat poison. Later on, it was discovered that warfarin could be used to prevent certain types of blood clots in humans when used in low doses.
Warfarin is an effective but dangerous drug
Warfarin is a very effective anticoagulant drug. It is also one of the most dangerous medicines that is used. This is because the correct dose varies substantially between patients and, to make things even more complicated, over time for the same patient.
Thus, treatment needs to be strictly monitored to avoid over- or underdosing the drug. If a patient is overdosed with warfarin it will result in excessive bleeding, while underdosing leads to reduced blood-thinning efficacy and risk of blood clots.
There are many factors that affects the efficacy of warfarin. Changes in diet, illness and initiating treatment with other drugs may affect the blood-thinning properties of warfarin.
Drugs that increase the metabolism of warfarin will lead to lower concentrations of warfarin in blood and thus a lower blood-thinning effect, in turn leading to increased risk of blood clots forming. These examples highlight the complex task of maintaining effective blood-thinning effect with warfarin and why extensive drug development efforts have been aimed at discovering safer alternatives to warfarin treatment.
Desire to create more knowledge about the effect of warfarin
The variability in the blood-thinning effect of warfarin obviously presents a substantial problem for the patients and we wanted to obtain more information about this.
We used data from 7,000 patients and their ‘INR-values’ which is a measurement of the blood-thinning effect of warfarin. A high INR indicates 'thin' blood while a low indicates ‘thick’ blood.
We were able to match this data to other data sources covering which other drugs the patients were using, and importantly when they started using them. From this, it should be easy to decide what drugs would make the INR-value rise or fall. Or so we thought…
Nonsense results
In theory, the idea was good, but it did not work in practice.
After a year of work and multiple attempts to refine the models, we had to give up. No matter what we did the results would not make sense.
According to our results, drugs we knew should not affect warfarin all appeared to have a noticeable effect. And drugs associated with high INR-values (thin blood) would also consistently look like they were related to low INR-values (thick blood).
This was immensely frustrating because it did not make any sense.
A cup of coffee led to a breakthrough
Anton and our colleagues wrote an article about the research project to at least obtain something for all our hard work. The article got accepted for publication in a methods journal by the end of 2014.
Our hope was that this could save other researchers the effort if they have had the same idea. On this note we had ended the story. Or so we thought.
Six months later, Anton and Tore went for a cup of coffee. In the meantime, Tore had started his PhD project. He did not work with large amounts of data, like Anton. Instead, he worked with smaller clinical trials. Tore commented that he had read the article but had not understood a word of it.
It was, admittedly, quite complicated.
He did not understand why the models had to be so complicated. Why did Anton not use simpler methods, such as those implemented in smaller clinical trials – perhaps something similar to what he did when he analysed the blood samples from his healthy volunteers?
Tore suggested to simply use the last INR-value before starting another drug and compare this to the first INR-value after initiating the new drug. Theoretically, it would make it much easier to determine whether it affected the blood thinning capabilities of warfarin.
While Tore explained his simpler setup, Anton smiled. This was too simple. But how could Tore know any better when he did not have experience in working with registry data?
However, Tore’s suggestion kept nagging Anton. There was no obvious reason for why Tore’s suggestion should not work. One Friday afternoon Anton surrendered and reluctantly he started to write the code.
Due to the simplicity, it did not take long and after working all weekend it became clear that Tore was right. Eureka! Suddenly, all the confusing signals were gone. Now, all Anton needed was to demonstrate that the method worked.
Dicloxacillin worked
Using the model to confirm an already known effect would provide compelling evidence that the setup was working. A vague memory came to Anton’s mind. He recalled that a clinician once told him that dicloxacillin, an antibiotic used to treat skin infections, would lead to lower effect of warfarin.
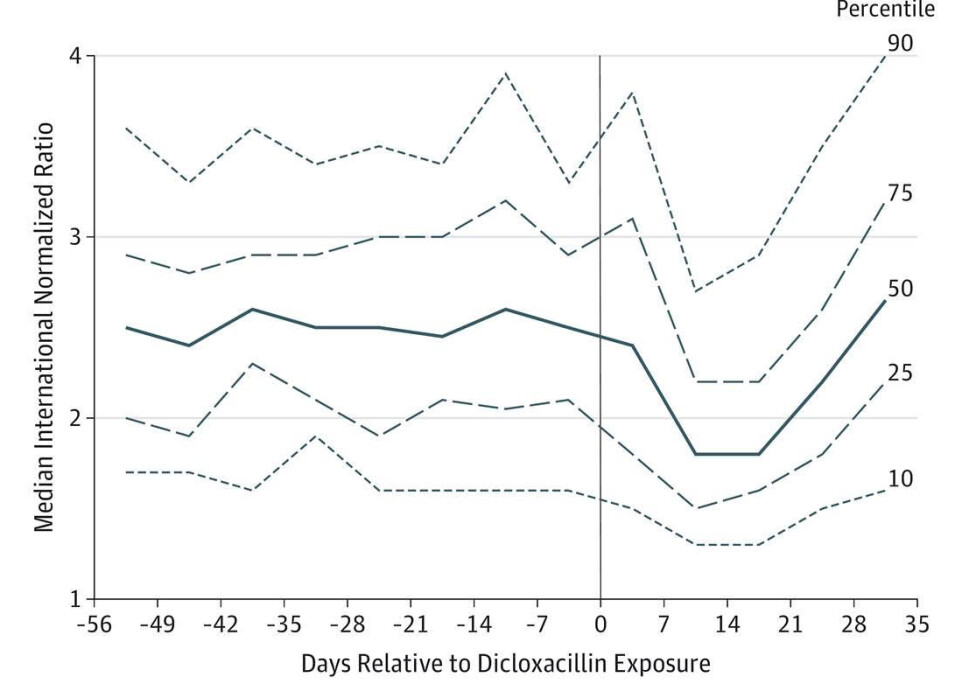
He therefore used the model to test dicloxacillin. The excitement was noticeable when the result clearly showed that initiating dicloxacillin led to substantially lower effect of warfarin.
The graph was soon sent to Tore, along with an apology from Anton for not immediately realising the ingenious in his suggestion. Tore was quick to respond. He ignored the apology and asked why on earth he had chosen to run dicloxacillin?
Anton was quite sure that this was well-known, but when he tried to look the drug up, it was rarely mentioned as a complication. In fact, data describing this was almost non-existing. The only data he could find seemed to be three case reports – stories about a few patients that had experienced a drop in their INR-value, all from the late 80’s.
It seems likely that the Danish physician, possibly from Odense University Hospital, had experienced something similar with a patient and told a group of young pharmacy students about this. Unfortunately, we cannot acknowledge this physician, as we actually do not remember who it was.
Suddenly we both had a working model and a ‘smoking gun’ showing a hitherto unknown connection between dicloxacillin (a rather commonly used drug in Denmark) and decreased effect of warfarin.
We generated new knowledge by coincidence – but also new questions
We quickly gathered a small team and dropped what we were doing to write a short report. Five days later it was sent to one of the most recognized medical journals, Journal of the American Medical Association (JAMA).
The paper was accepted for publication and this happy coincidence allowed us to contribute to understand why and how warfarin sometimes stop working in patients. But as it often is with research, one answer raises two additional questions.
In this case we wondered why warfarin stops working when a patient is treated with dicloxacillin? And importantly, does it actually have consequences for the patients?
Liver cells in San Francisco and 10 million Danish crowns
In the meantime, Tore was preparing for a postdoc – a research position after the PhD. This project is usually performed abroad to contribute with new knowledge, skills and perspectives and to establish collaborations that can be brought home.
Tore went to USA where he planned a one-year stay in a high-profile laboratory in San Francisco. In this laboratory, he met Cyrus Khojasteh who was working at Genentech in South San Francisco. Cyrus had an appropriate model of liver cells that could be used to understand what was causing this remarkable drop in effect of warfarin when dicloxacillin was initiated.
The liver was the prime suspect, as this is where most drugs are metabolised. At the same time, a clinical study in healthy volunteers was done in Denmark.
While Tore was enjoying himself in USA, Anton and his colleagues enjoyed themselves with the model. Because the model new allowed us to look into many other unanswered questions.
In one article we disproved the hypothesis that drugs against stomach acid would affect the efficacy of warfarin. In another article we showed that warfarin patients with a fungal infection in their mouth had to choose treatment with nystatin over the frequently used drug miconazole, as treatment with miconazole would lead to a considerable increase in INR values.
Furthermore, the model was used to identify brand new hypotheses that would lead to Tore obtaining a major research grant for 10,000,000 Danish crowns – but this is another story and we need to get back on track.
Swedish data led to new answers
After one year ‘over there’ Tore was ready to return home. He had published an article which showed that dicloxacillin increases the amount of enzymes that are responsible for metabolising warfarin.
In a small clinical study in 12 healthy volunteers, he even showed that the effect was very significant and applied not only to dicloxacillin but also to how many other drugs are metabolized. As a bonus insight he also showed a comparable but smaller effect for flucloxacillin, an antibiotic related to dicloxacillin which is commonly used internationally.
The flucloxacillin effect was interesting to study in a petri dish but it was still unclear whether this effect would matter. We could not use Danish data as flucloxacillin is not widely used in Denmark. However, this is not the case in Sweden, where flucloxacillin is the only drug used of the two.
Tore chaired a conference in Nyborg, Denmark in the summer of 2018, where he got in touch with a researcher from Sweden who had access to Swedish INR-data. Six months later this was analysed and published as an article. Together with the Swedish researchers, we found a similar but less marked effect of flucloxacillin on warfarin’s blood-thinning capabilities.
This meant that the first question was now answered: now we knew how dicloxacillin (and flucloxacillin) increase the metabolism of warfarin.
Does the effect mean anything to the patients?
We are now approaching the finish line, but we still need an answer to the second question: does all of this even have consequences for the patients?
We know that the blood gets thicker for a brief period of time after initiating dicloxacillin. However, if this does not lead to an increased risk of blood clots, then the knowledge we spent years generating is actually an academic exercise with little importance to clinicians and patients.
To answer this question, we gathered our research group where we all had different specializations and research experiences. To understand this, we again turned to the world class Danish health registries.
We found more than 100,000 Danish patients using warfarin from 1995 up to 2017 with information about which drugs these patients were prescribed. Cross-linking this data with information on when and which type of blood clot the patients experienced during treatment, we were able to assess risk of serious blood clots after dicloxacillin was prescribed.
We showed that the risk of a blood clot was almost doubled for 21 days after initiation of treatment with dicloxacillin – which we published as an article in one of the leading journals for drug research.
A random find could be used to something
A risk twice as high sounds dangerous but it is important to remember that the risk of a blood clot within a 21-days interval is fairly low. To more accurately reflect this, the number needed to harm can be calculated. This number reflects how many patients need to use both drugs in order for one extra blood clot to appear.
For dicloxacillin, this number was approximately 1,000. An extra clot in one of thousand patients is -fortunately- not a lot. But importantly, it is a blood clot that is easily preventable now that the knowledge has been generated.
For some infections we can choose another antibiotic. If a patient needs to use dicloxacillin or flucloxacillin we now know exactly when warfarin will become less efficient. This means that we can adjust the dose to counteract the problem and thereby protect patients.
Many questions still remain regarding the role of dicloxacillin and flucloxacillin in causing therapeutic failure of other drugs. Theoretically, drugs against epilepsy could also be affected. And what consequences can this bring patients with epilepsy?
With a new research grant from the Novo Nordisk Foundation this is some of the questions we want to look into the next couple of years. Thus, the research-wheel keeps spinning.
At the end of the day, this new knowledge is based on a small Danish warfarin-register and two hopeful PhD students in 2012. This led to American petri dishes, Swedish warfarin-patients and back to the Danish health registries. It also highlights once again that sometimes (maybe even often) science happens by pure coincidence…
Read the Danish version of this article on Videnskab.dk’s Forskerzonen.






