Researchers' Zone:
How smart antibodies can lead to superior treatments
Antibodies are the garbage trucks of the human body. They take the stuff we do not want in our system and drive it off to the waste dump. This system is smart, but it can be improved.
With COVID-19 still raging around the world, many of us have probably heard about antibodies recently.
This could either be as antibody tests, which are used to determine if a person has previously been infected with the coronavirus, or as antibody therapies used to treat people infected with the virus.
But what are antibodies?
Antibodies are a natural part of our immune systems and are produced by the white blood cells in our body. Their job is to battle viral, parasitic, and bacterial infections that we encounter throughout our lives.
But today we can also produce antibodies outside the human body by using modern biotechnology that mimics the human immune system.
And since the first antibody was approved for use as a pharmaceutical in 1986, antibody treatments have been so successful that in 2018, 7 out of the 10 most sold drugs worldwide, measured by revenue, were antibodies.
These antibodies can be used to treat diseases that are otherwise difficult to treat, such as COVID-19, cancer, arthritis, or multiple sclerosis.
And by developing our own antibodies we can improve the natural system. But how do we do this exactly? This, we will explain shortly.
But first we must look at the natural antibodies to understand how they work and how the system can be improved.
What makes antibodies so special?
Antibodies are special because they bind their targets (called antigens) with ultra-high specificity. Antigens are often parts of the virus, parasite, or bacteria that can be recognized and bound by the antibodies.
Through this recognition and binding, the antibodies can neutralize the antigens or mark them for destruction by the immune system.
When antibody tests are performed for the coronavirus, the patient is being tested for antibodies circulating in their blood that recognize the coronavirus.
If the blood contains such antibodies, it is evidence that the patient’s white blood cells have produced antibodies that target the coronavirus, and therefore, they likely fought off an infection recently.
How antibodies can be almost too good for their own sake
Antibodies are ultra-specific in what they bind. This is necessary for them to function, so they do not accidentally bind to elements of our own cells and then mark them for destruction.
On top of their binding being very specific, it is also a strong binding, meaning that they do not let go of their targets once they have found them. However, the binding strength of antibodies can be considered both their biggest strength and their biggest weakness.
Strong binding is great when you, for example, want to ensure that a virus remains neutralized and will be prevented from exerting harm. But if the antibody holds on to that one antigen, it also means that one antibody can only neutralize one antigen.
This leads to the therapeutic potential being lower than it theoretically could be for each antibody, which means that a larger number of antibodies are required, resulting in increased costs of treatment.
To simplify, you can think of the antibody system like a garbage truck picking up a load of garbage. The antibody is the truck, and the antigen is the load of garbage.
But this is not a very smart garbage truck. Therefore, once it has picked up its load, it will drive around and never get rid of it until both the garbage truck and the garbage load are thrown into the dump.
Would this hypothetical garbage truck not be much more useful if it could pick up the garbage, deliver it to a location, dump it there, and then go back to picking up more garbage from elsewhere? Of course it would.
Recent developments in antibody research have allowed scientists to develop antibodies with precisely this function.
How smarter antibodies can be developed
The trick to these smarter antibodies is to design antibodies that can not only bind to, but also release their antigens under specific conditions.
The most challenging part is figuring out when the antibody should release the antigen. If this part is just a little off, we risk that the antibody releases the antigen too early, which could result in the antigen being able to cause further harm in the human body.
One way of accomplishing this is to make the binding dependent on the pH in the body. This is because different environments in the body have different pH levels.
In recent years, scientists have developed smart antibodies, that are affected by these changes. These smart antibodies function by having one area with one pH designated as the binding zone (the location where the garbage truck picks up the garbage) and one area with another pH as the drop off point (where the garbage truck delivers the garbage).
Introducing the body’s own antibody recycling system
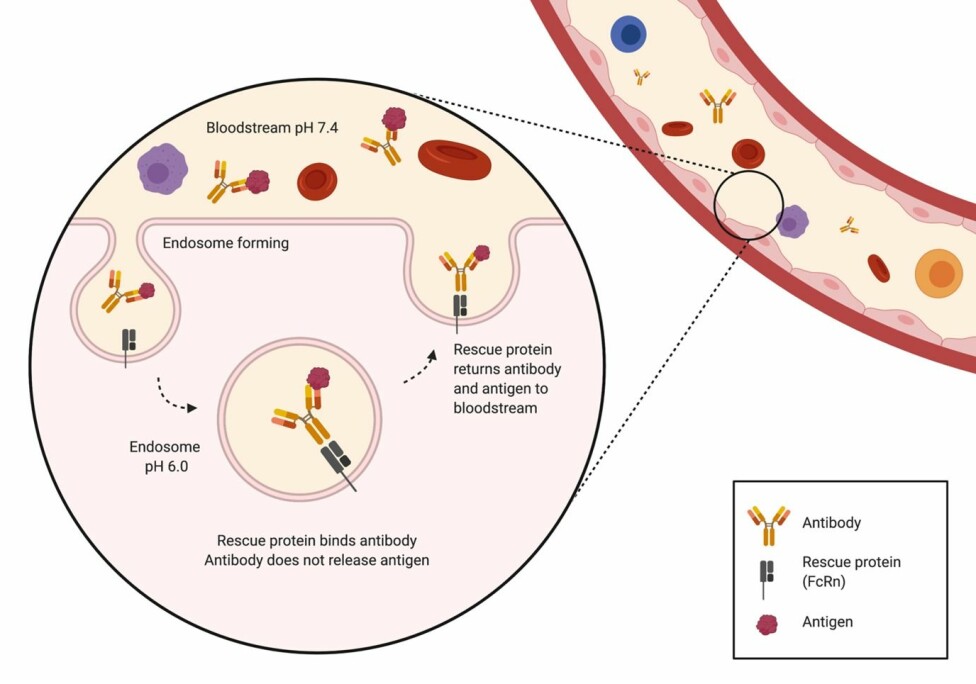
In the body, antibodies are regularly transported inside the cells from the bloodstream in something called endosomes. These are spheres made of the cell’s own membrane that can be transported into the cell.
As the endosomes are closed containers, they don’t expose anything inside the cell to their harmful contents. The endosomes then fuse with a different type of endosomes, called lysosomes, which are similar structures, but with a much lower pH. The lysosomes contain enzymes that degrade the contents of the endosome.
However, they do not carry this out before the body has had a chance to rescue the useful antibodies from being broken down.
This rescue is accomplished by rescue proteins (FcRn) that bind the antibodies to the inside of the endosome. Here they prevent the antibody from entering the harmful lysosomes and instead bring it back to the surface of the cell, where the antibody is released again.
Basically, what this means is, that the human body has its very own functioning recycling system, and that we can use it to sort, recycle, and dispose of the antigens the antibodies bind to.
However, the problem occurs when the antibody is too tightly bound to the antigen. In this case, the antigen is also returned to the outside of the cell along with the antibody, meaning the antibody is not free to bind more antigens (the garbage truck is full and cannot pick up any more garbage).
But here comes the good part. The pH in the bloodstream where the antibodies are usually found is almost neutral (pH is 7.4); however, inside the endosomes, the pH is more acidic (pH is 6.0).
Suppose we could create an antibody that could bind an antigen outside of the cell (in the bloodstream) at pH 7.4 and let go of it once inside the endosome, where the pH is 6.0. In this case, the antigen would be degraded, while the same antibody could be reused many times (the garbage truck can dump the garbage and go pick up more).
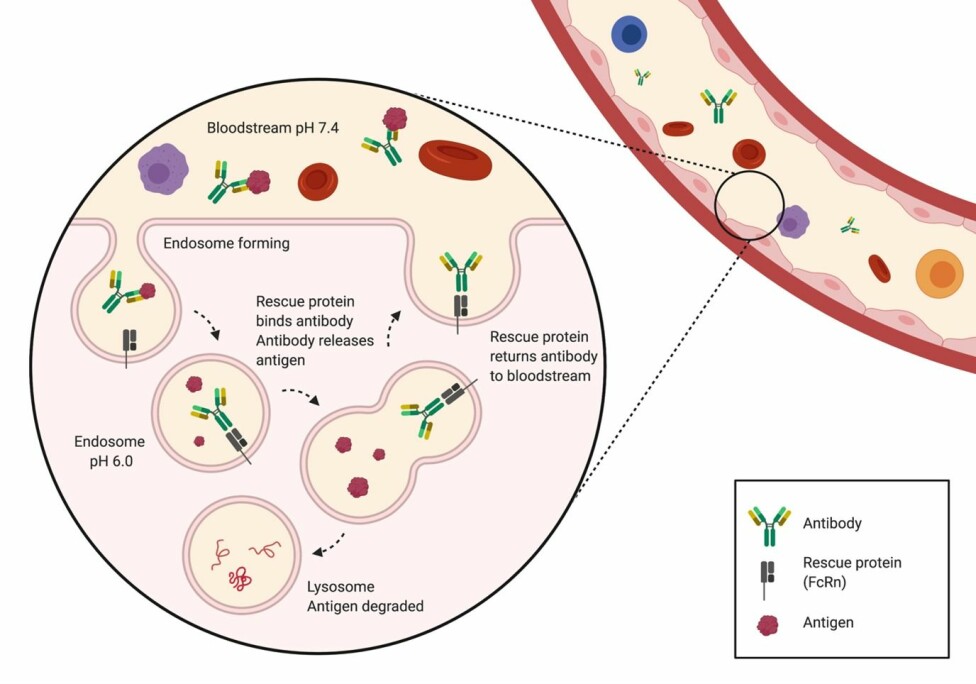
Does this sound like science fiction? It is not.
How can we develop this?
The future is not bleak for antibody therapies. Antibodies with this exact function have recently been demonstrated to work by scientists.
The scientists showed that they could develop antibodies that bind an antigen at a physiological pH and release the antigen at a more acidic pH – precisely the conditions needed for being rescued in the endosomes through the rescue protein (FcRn).
These antibodies were discovered using a Nobel-prize winning method called phage display technology. This technology allows scientists to reliably obtain antibodies targeting new antigens, allowing them to develop potent therapies for a wide range of disorders and conditions.
What could the future look like?
The development of these smart, pH sensing antibodies has the potential to revolutionize treatments of a vast number of conditions. One such condition is snakebite envenoming, which predominantly affects people in low-income countries with little access to expensive treatments.
If we could develop smart antibodies to treat snakebite envenoming, each antibody could be many times more efficient than in current treatments, potentially bringing the cost down dramatically.
Turning the tables, this technology could also be used to make antibodies that do not bind their antigens at a physiological pH but instead bind at a lower pH.
These antibodies could be used to target disorders such as cancers, where the cancer cells can be almost indistinguishable from healthy cells, but where they thrive better in environments with a low pH and thus can be targeted with smart antibodies.
Smart antibodies have the potential to improve the lives of many people around the world by creating better and more affordable treatments, so we are definitely not done hearing about smart antibody treatments.
This article is also published in Danish. Read the Danish version at Videnskab.dk's Forskerzonen.
References:
- Rasmus Wied Pedersen's profil (LinkedIn)
- Line Ledsgaard Jensen's profile (DTU)
- Charlotte Rimbault's profile (DTU)
- Christoffer Vinther Sørensen's profile (DTU)
- 'Sweeping antibody as a novel therapeutic antibody modality capable of eliminating soluble antigens from circulation', Immunological Reviews (2016), DOI: 10.1111/imr.12392
- 'De novo isolation of antibodies with pH-dependent binding propertie', Immunological Reviews (2016), DOI: 10.1111/imr.12392
- 'pH-responsive antibodies for therapeutic applications', Journal of Biomedical Science (2021), DOI: 10.1186/s12929-021-00709-7





