Researchers' Zone:

Extreme variation in snake venom: no two bites are the same
Snake venom is affected by many factors and therefore varies to a degree that makes it almost impossible to find broadly effective treatments.
Throughout history, snakes have achieved an arguably iconic status in popular culture due to the deep fascination and primal fear they inspire.
As of today, these reptiles are still revered on the one hand and reviled on the other, not least because of physical features that make them stand out (or slide low) to our perception: legless bodies, forked tongues, and a skin entirely covered in scales. Then, of course, there is venom.
Although only around 300 of the over 3,500 snake species known to science possess dangerous venoms, their undeserved reputation as bloodthirsty killers is often extended to the entire lineage.
With this in mind it is no wonder that snake venom has received plenty of attention from the scientific community, with thousands of studies and hundreds of books discussing its evolutionary history and ecological role as well as the molecular mechanisms behind its devastating actions.
The mystery of snake venom
Nowadays, it is well known that snake venom is a mixture of toxic proteins and enzymes that allowed the species that developed it to take down prey through chemical means, which in turn led to an explosive radiation of venomous snakes all over the world.
However, venom is not a one-size-fits-all trait: as snakes diversified to colonize all kinds of habitats, so did the composition and activity of their venoms. In fact, several factors related to the snake’s own biology as well as the surrounding environment have shaped (and continue shaping) the evolution of toxins into a myriad of different types.
As we will see, such variation is often found even within single species and poses a great challenge for the development of effective antivenoms. But first, let us dive into the complex world of snake venom evolution.
A mosaic of venoms
The primary role of snake venom is prey incapacitation, which requires toxins that can paralyze or kill the prey as quickly as possible with minimal effort.
Several studies reporting ‘fine-tuning’ of venom activity against the snake’s preferred prey through natural selection have been published in recent decades, supporting the crucial role of diet as an evolutionary driver for the toxic arsenals of these reptiles.
That being said, other influencing factors are also at play in shaping venom characteristics, such as local environmental conditions (e.g., temperature, elevation, seasonality) and phylogenetic relatedness (closely related species often tend to have similar venom characteristics).
While this might well appear straightforward when talking about different snake species, an intriguing aspect of the intricate variability in venom profiles is that it is just as widespread even within single species.
What’s on the menu? Diet as a driver of venom variation
But why would venom composition and/or activity differ between populations and even individuals of the very same species? Ultimately, the same factors that drive between-species venom variation are at play at the within-species level.
Evidence of this was first described in the Malayan pitviper (Calloselasma rhodostoma), whose venom displays considerable variability throughout the species’ range in South-East Asia according to different dietary preferences between separate populations.
Thus, frog-eating pitvipers diverge widely from rodent specialists in terms of venom composition despite belonging to the same species, indicating a strong influence of preferred prey type on venom.
On the other side of the world in the Amazon rainforest, the common lancehead (Bothrops atrox) displays a similar pattern; individuals preying on amphibians in humid and densely vegetated regions possess a more highly procoagulant (blood clot-forming) venom than their savanna-dwelling cousins that mainly feed on mammals and/or other reptiles.
For the frog specialists deep in the forest, a more potent venom would likely be helpful in incapacitating prey before they can escape in the water or in the forest’s understory, maximizing the odds of a successful hunt for the snake.

Coming of age: Changes in venom between life stages
Venom profiles can also change drastically with age: while the common assumption that venom is invariably more potent in juvenile snakes than in adults is a misconception, sometimes it does hold true – If not to humans, to prey species.
The ringed brown snake (Pseudonaja modesta) is the only exception to this as it retains the neurotoxic type into adulthood, likely due to its continuing to prey on reptiles and amphibians throughout its life.
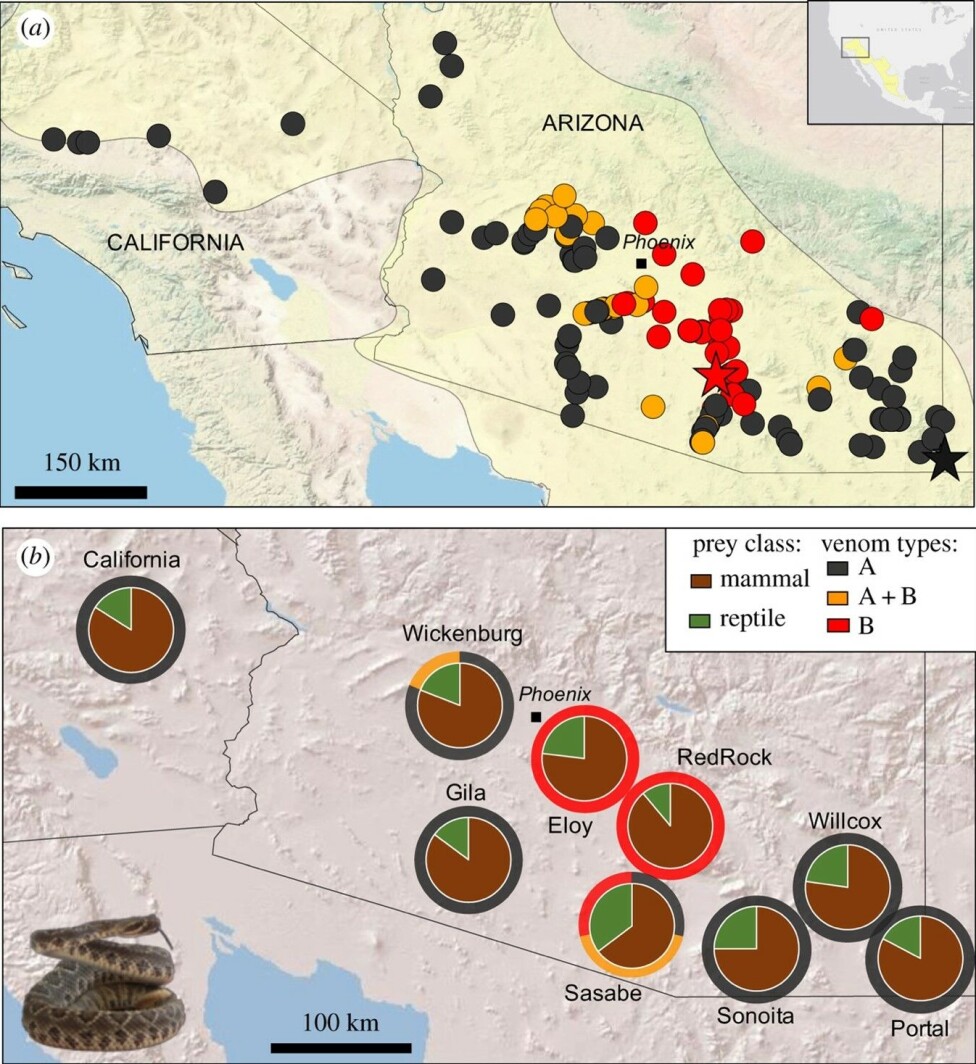
Environmental influences on venom variation
In fact, some populations of this species are known for the trademark Mojave-toxin (MTX), a highly neurotoxic protein that causes paralysis and respiratory failure by blocking neural transmission to the muscles; conversely, other Mojave rattlesnake populations display a markedly blood-affecting venom, with symptoms mainly revolving around internal bleeding and local tissue damage.
These two profiles, commonly termed type A and type B respectively, can even merge in certain geographic regions (type AB).
Intriguingly, the presence or absence of several toxins – including MTX – across populations strongly correlates with factors such as temperature fluctuations and seasonal precipitation patterns but not with diet, which means that diet is not the only factor driving venom variation.
Venom variation can be caused by a combination of factors
As if the topic wasn’t already complex enough, differences in venom composition have been documented even on a single-individual basis and within newborns from the same litter in certain species.
Nature can rarely be split into neat, independent compartments, and snake venom variation, be it between or within species, is no exception: more often than not, venom composition and activity are shaped by the combined selection pressures of multiple influencing factors.
The Northern Pacific rattlesnake (Crotalus oreganus), commonly found in California, is a perfect example of this.
Rattlesnake venom reaching unknown heights
This species was long thought to be engaged in an ‘arms race’ with one of its preferred prey items, the California ground squirrel (Otospermophylus beecheyi), many populations of which are resistant to the snake’s venom.
However, recent research has shown that differences in the composition of the Northern Pacific rattlesnake's venom are also correlated to other limiting factors such as elevation due to shifting environmental conditions at different heights.
Thus, the concept of the rattlesnake-squirrel arms race was expanded to include the profound effects of local geographical variables – a scenario now accordingly described as ‘phenotype matching’ to a combination of influencing factors.
The Northern Pacific rattlesnake case shows once more that venom characteristics are the result of a complex interplay of variables, making it difficult to talk about snake venom in terms of broad types and categories even when it comes to single species.
Significance of within-species venom variation
Such an intricate pattern of selection pressures and adaptations is a treasure trove of discoveries for evolutionary biologists.
However, it is just as much the bane of clinicians and antivenom manufacturers because it makes it almost impossible to create broadly effective antivenoms.
In fact, snakebite is a major public health issue, which is further worsened by extensive venom variation within species in the same geographical region. To understand why this is the case, and how science can solve these challenges, we need to delve into the tragedy that is the global snakebite emergency.
The snakebite challenge
Despite scarce attention from mainstream media, snakebite is nothing short of a global health crisis: as many as 138,000 people per year lose their lives to snake envenoming and over 400,000 suffer permanent physical and/or psychological disability.
The only specific treatment for snakebite is antivenom, which can be either monovalent (i.e., single-species specific) or polyvalent (effective against venoms from multiple species).
However, antivenom is often unavailable due to short supply, unaffordable for the patients who most need it, or ineffective because of poor neutralization efficiency stemming, among other causes, from the current process for antivenom manufacturing.
Overall, the antivenom production pipeline hasn’t changed significantly since the first snake antivenom was developed by eminent French microbiologist Albert Calmette in 1894.
Flaws in antivenom production result in poor efficacy
While recent advances have greatly enhanced the effectiveness of antivenoms, certain issues still need urgent solutions.
These issues range from the risk of allergic reactions to equine and ovine antibodies to poor efficacy of the antivenom against snake species or populations that are not included in its immunizing mixture (i.e., the species whose venom was used in producing the medicine).
Besides, the vast majority of antibodies obtained from the immunized animal’s blood are targeted towards toxins of large size that are more easily recognizable by the immune system.
However, these are often not the (most) lethal elements in the venom, which are frequently small and therefore able to evade recognition by antibodies.

Venom variation reduces antivenom effectiveness
Within-species venom variation adds even further to the antivenom efficacy crisis.
The case of India is notorious in this sense: of the over 50,000 annual snakebite casualties reported in the country, most are attributable to those species known as the ‘Big Four’:
- the Indian cobra (Naja naja)
- the Russell’s viper (Daboia russelii)
- the common krait (Bungarus caeruleus)
- the saw-scaled viper (Echis carinatus).
All are known to display venom variability to different extents throughout the country, meaning that the antivenoms produced from venom collected in any one region are often ineffective, if not entirely useless against venoms from other (distant) regions.
This translates into a higher risk of death or permanent disabilities for snakebite patients and makes appropriate treating of envenoming cases incredibly challenging for doctors.
To tackle this major obstacle, the WHO guidelines for antivenom production released in 2016 recommend the collection of snakes from multiple regions and of all life stages for any given species to obtain a representative sample of venoms for antivenom production.
While implementation of this strategy will undoubtedly improve antivenom effectiveness, science might have another trick up its sleeve to mitigate the impact of venom variation on snakebite treatment.
Calmette’s dream is dead, but hope lives on
As we have seen, long gone are the days when Calmette in 1894 triumphantly proclaimed that his newly developed monovalent antivenom sourced from monocled cobra (Naja kaouthia) venom would potentially be able to neutralize any snake venom known to man.
In response to these pressing issues, recent research has explored the possibility of replacing animal-derived antibodies with selected human ones able to neutralize highly toxic venom components that are common to many different snake venoms.
For instance, one or a few such antibodies would ideally be able to bind to a wide array of snake venom metalloproteases (SVMPs), which are a hallmark component of many viper venoms causing extensive blood clotting disorders and tissue breakdown.
Others would instead be geared to target three-finger toxins (3FTx), a class of mostly paralysis-inducing proteins that attack the nervous system and are common in elapid snakes such as cobras.
No one-size-fits-all treatment for snakebite
Thus, an appropriate mixture of toxin-family-specific human antibodies would be able to neutralize a wide array of venom toxins regardless of between- and within-species variation, potentially leading to groundbreaking progress in the treatment of snakebite envenoming.
Although such antibodies are still to be clinically tested to confirm their efficacy as antivenom products, optimism is warranted as even antibodies with narrow specificity against one or a few toxins have yielded promising results in mice so far.
Over a century after Calmette’s premature enthusiasm in the quest for a universal antivenom for snakebite, a better understanding of the dynamics and implications of venom variability has taught us that his dream was in vain.
The development of a one-size-fits-all antivenom is as likely as finding the pot of gold at the end of the rainbow.
Nevertheless, acknowledging this might enable us to feasibly replace the century-old and sub-optimal treatment with carefully designed next-generation snakebite therapeutics that could save many more lives of envenomed victims every year.
Read the Danish version at our sistersite Forskerzonen.
References:
Lorenzo Seneci's profile (DTU)
Timothy Patrick Jenkins' profile (DTU)
Christoffer Vinther Sørensen's profile (DTU)
'Diet and snake venom evolution', Nature (1996). DOI: 10.1038/379537a0
'Snakebite envenoming — A strategy for prevention and control', WHO (2019)
'Venom, antivenom production and the medically important snakes of India', Current Science (2012).




