Huge X-ray laser reveals new molecular state
Using a specially designed X-ray laser, researchers have managed to photograph what happens when molecules bind to or unbind from the surface of a catalyst. The findings may be an important step in the development of new forms of renewable energy.
Petrol, plastic bowls, cream, rain jackets, cleaning products, fertilisers, biofuels, beer, plastic bags, water bottles…
Lots of everyday objects are made with catalysts – a substance that helps activate chemical reactions without being converted or used in the reaction.
But how do these catalysts actually work?
A research group has now, as the first one ever, captured photographs of what happens when molecules bind to or unbind from the surface of a catalyst.
”We found that the molecules can, for an extremely short period, be in a special state, which is located farther away from the catalyst surface than we had expected,” says Jens Nørskov, a Danish professor of Chemical Engineering at Stanford University, US, who is one of the researchers behind the new study.
“A vast majority of chemical processes are controlled by catalysts, which is why it is absolutely crucial that we understand how they work.”
X-rays function as a camera
Since the chemical process is extremely fast, the researchers needed a special instrument – a so-called free-electron X-ray laser.
“The instrument is an unbelievable piece of engineering and it is the first one of its kind in the world. A linear accelerator stretches three kilometres up into the mountains behind me here at Stanford University.”
We found that the molecules can, for an extremely short period, be in a special state, which is located farther away from the catalyst surface than we had expected.
Jens Nørskov
In this long accelerator, electrons are accelerated in a special way that makes them emit strong X-ray radiation. This radiation can be used as a type of camera, which can take pictures of tiny molecules with extremely short time intervals.
Ruthenium as a catalyst
The researchers used the free-electron X-ray laser to study the catalyst ruthenium, a chemical element that resembles iron and is used as a catalyst in a number of processes, for instance in converting wind and solar energy into usable energy.
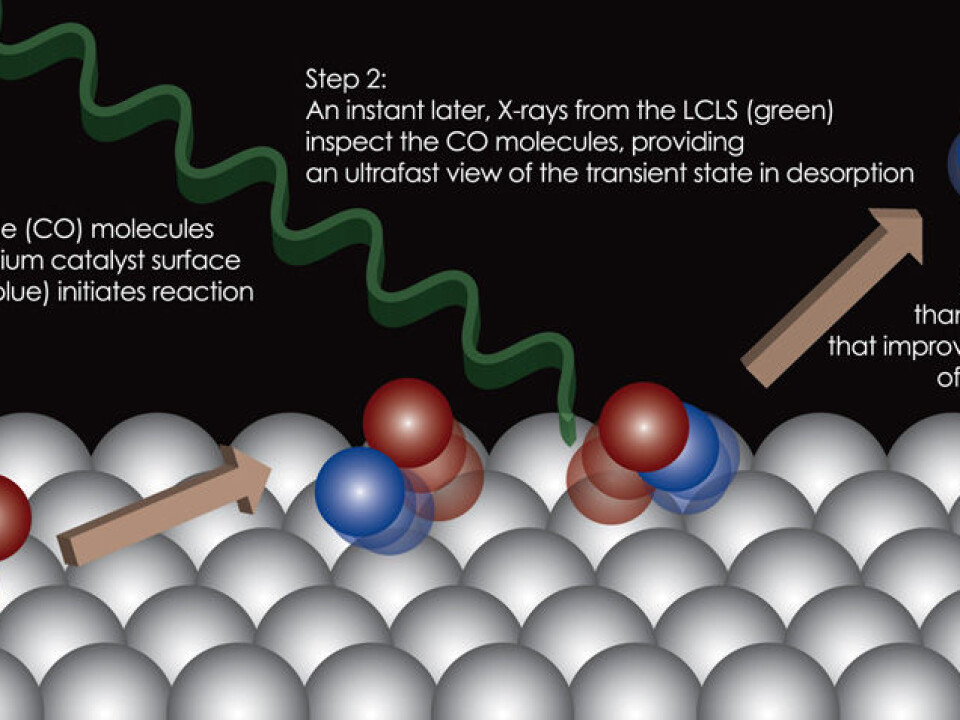
One of the molecules that often use ruthenium as a catalyst is carbon monoxide (CO). The CO molecule is typically found in the shape of gas, and when the gas molecules enter into a chemical reaction, they ‘fly’ in and bind to the ruthenium surface.
Ruthenium now functions as a catalyst that speeds up the reaction, and after a short while the molecules can fly away from the ruthenium again, and the reaction is complete.
The researchers irradiated the CO molecules with a laser pulse to get them to react and leave the ruthenium surface after they had bound to it in the normal way. The X-rays from the free-electron laser kept a close eye on this detachment process.
”So you could say that we have filmed the process, even though it takes place over a very, very short time,” says Nørskov.
Molecules enter a strange transition state
Having analysed the footage, they could see that the CO molecules behaved strangely when moving away from the ruthenium surface. The molecules appeared to be in an entirely new state – between being stuck to the ruthenium surface and drifting around as free gas molecules
Such ‘transition states’ for molecules were already suggested by Nobel laureate Irving Langmuir in the 1930s. But this is the first time it has been demonstrated experimentally, explains Professor Jane Hvolbæk Nielsen, of the Technical University of Denmark, who praises this attempt at combining an innovative experimental design with advanced theory:
”It has previously not been possible to measure this state,” she says.
“It’s a state in which the CO molecule is loosely attached to the surface and can move relatively freely. This study shows that the molecule has a small refuge where it can take a break – either on its way toward or away from the surface. And that has an effect on how fast a chemical reaction can occur.”
Although the experiment only used CO molecules with ruthenium as catalysts, Nielsen believes it is likely that other molecules and catalysts would behave in the same way, because this is a quite ordinary molecule and an ordinary metal surface.
She believes many other researchers will now start investigating whether the same applies to other similar systems and catalysts.
------------------------------
Read the Danish version of this article at videnskab.dk