A new way of making complex anticancer compound
Ingenol, a substance known for its anticancer potential, has so far been extracted from plants, but now scientists have achieved the first efficient chemical synthesis of the substance.
To farmers and gardeners, the petty spurge is just a weed that needs to be removed from the fields.
But to the pharmaceutical industry, the plant has long been in the spotlight because of its so-called Ingenol substances, which have shown promising results in the fight against cancer.
The problem is that the petty spurge only contains tiny amounts of these special substances, and extracting and refining them from the plant is a costly and ineffective process.
However, scientists have now managed to produce Ingenol synthetically in the laboratory.
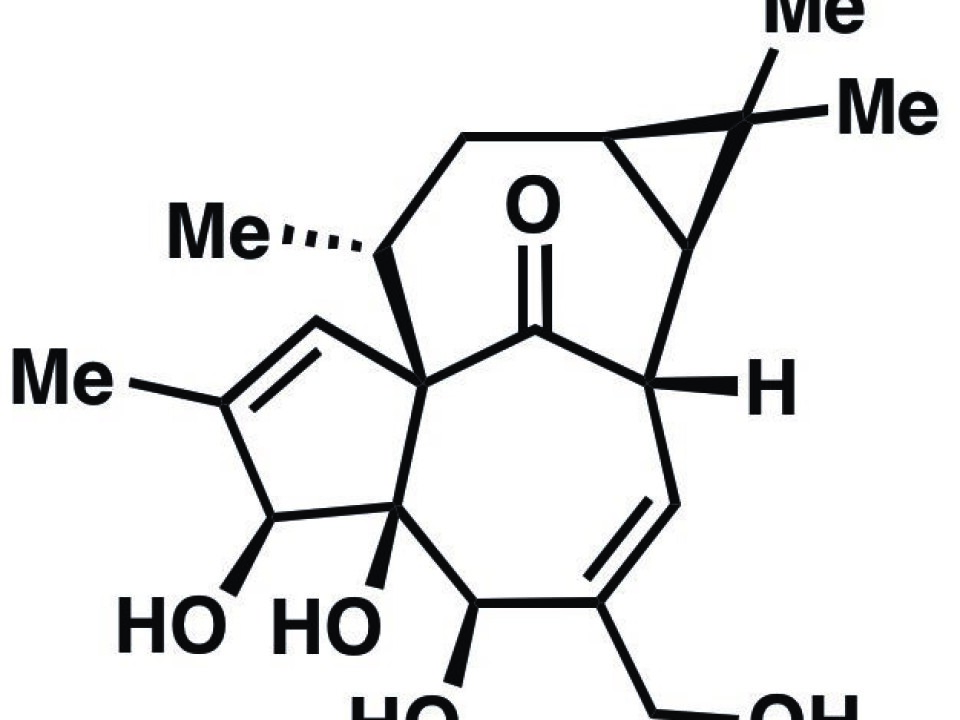
“Ingenol has a highly complex structure, and that makes it very hard to produce it chemically. Most organic chemists would probably not have expected that it would be possible to do it as effectively as we have done in our study,” says lead author Lars Jørgensen, a Danish chemist at The Scripps Research Institute in America.
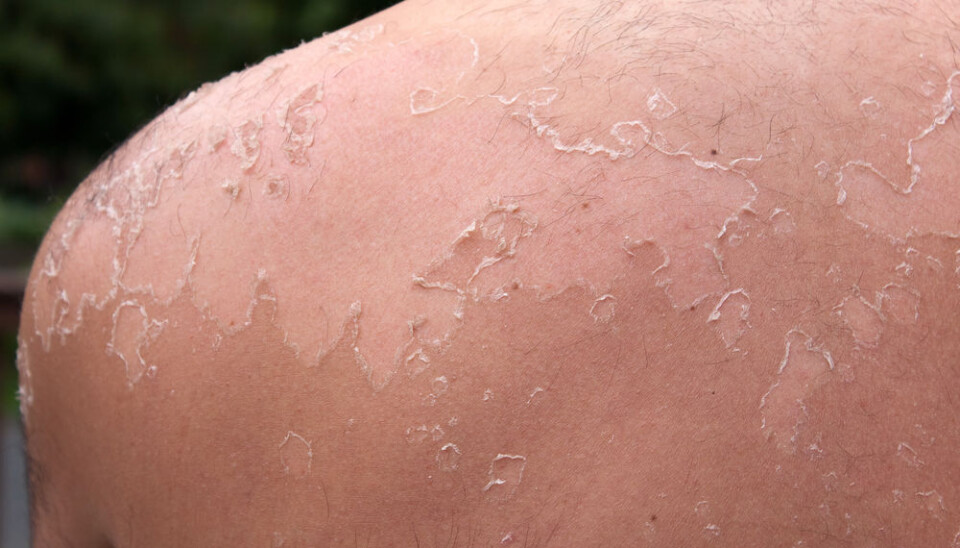
Ingenol can treat precancerous skin condition
So what is it that makes Ingenol so interesting? According to Jørgensen, derivatives of Ingenol have shown potential in the treatment of the precancerous skin condition actinic keratosis.
Ingenol metubate, a derivative of Ingenol, already exists on the market and is FDA approved for the topical treatment of actinic keratosis.
Danish pharma giant uses the plant
This gel is manufactured by the Danish pharmaceutical company LEO Pharma, which extracts its Ingenol substances from the petty spurge plant.
Ingenol has a highly complex structure, and that makes it very hard to produce it chemically. Most organic chemists would probably not have expected that it would be possible to do it as effectively as we have done in our study.
”The substance that goes into this gel is extracted from plants, and this process is not only troublesome, it’s also expensive,” says the researcher.
”The plant does not contain much of the substance – for each kilo of the plant, you only get 1.1 milligram of Ingenol Mebutate. That’s why there has been so much focus on producing the substance synthetically in a lab, so that we’ll no longer be dependent on cultivation, harvest and so on.”
However, synthesizing a substance like Ingenol in the laboratory is far from a simple task.
How did they do it?
The Ingenol molecule is composed of the elements carbon, oxygen and hydrogen, but these three substances make up a very complex structure. This means that it is not enough simply to mix these substances in a flask and then create Ingenol from it.
”In terms of energy, these substances would assemble into a different structure from that of Ingenol. We therefore needed to take a detour, which forced the molecules to assemble in just the right way.”
This detour consisted of blending various chemicals with the ‘right’ chemicals to make them react with each other. The scientists ended up with a wide variety of substances in their flasks, but eventually they managed to blend and purify the chemicals in just the right way, resulting in the end product Ingenol.
Scientists mimic plants
”The strategy we used to solve this challenge was to look at how nature produces Ingenol – i.e. how the enzymes in the petty spurge produce the substance in the plant,” says Jørgensen.
When the petty spurge produces the Ingenol molecule, the plant’s enzymes first construct a skeleton of carbon and hydrogen. In the study, the researchers tried to mimic this process. They then added oxygen to the just right locations on the carbon framework to complete the synthesis, resulting in a molecule which had the right Ingenol architecture.
Method can be used in large-scale production
”We are actually not the first scientists to have synthesized Ingenol in the lab. But what makes our study special is that our method is far more effective, and now suddenly we’re seeing a potential for manufacturing Ingenol on a larger scale,” he says.
Other scientists have previously managed to produce Ingenol through a 37-step synthesis. In other words, they had to make a variety of substances react with each other 37 times before they ended up with the substance Ingenol.
The new study only used 14 steps.
Promising news for cancer patients
”Our process is more than twice as quick as previous processes, and there are probably a few researchers out there who have doubted whether it was even possible to produce Ingenol in this way. So this is a bit of a prestige project for a chemist,” says Jørgensen.
He explains that the next part of the project will consist of further developing the manufacture of Ingenol and to create new, similar substances that may be used in cancer treatment.
”By modifying the substance we may be able to produce a substance which binds more effectively to cancer cells or which may work in some other way. That’s what we’ll be looking into now.”
-----------------------
Read the Danish version of this article at videnskab.dk
Translated by: Dann Vinther