Researchers' Zone:
New silver-technology can track biomarkers for diseases like cancer
RNA-molecules’ behavior in cells can reveal early signs of serious diseases. The new technology makes RNA light up, allowing us to follow the life of the molecules.
RNA-molecules plays a significant role in developing serious illnesses like cancer. They can also detect whether a person has cancer and how the disease will progress.
So if we want to know if a person has cancer, it is a good idea to look at the life of RNA-molecules in cells.
But today we have to isolate the RNA from the cell if we want to look at it, which makes it impossible to see how the RNA works in the cell.
Visualizing the RNA in real-time in the cell can provide insights into the life cycle of RNA molecules and the cellular machinery involved in the development of serious illnesses.
But how can we watch the RNA live at work without isolating them?
Our research shows that the answer may lie in tiny clusters of silver particles that are encapsulated within DNA sensors.
Before we get to that, we need to understand why RNA is so important.
Role of RNA in cells and microRNAs
Imagine you have to follow a very long and detailed recipe. It's a bit confusing, so you ask ChatGPT to create a shorter version that only gives you the most important instructions.
Just like ChatGPT, RNA molecules send the most important instructions from your DNA to your cells so they can synthesize proteins to perform the right tasks.
MicroRNAs, a class of small RNA molecules composed of 20-25 nucleotide building blocks, perform a different role than longer RNAs composed of 100s of nucleotides.
MicroRNAs are crucial for gene regulation, influence around two-thirds of all human genes, and play pivotal roles in regulating various biological processes, including the development of diseases like cancer.
To perform their function properly, microRNA molecules are localized to specific parts of the cell where they perform critical functions.
Many RNA molecules are produced at specific times during a cell's lifecycle and their levels of expression can vary according to the cell's needs.
RNA is not static within the cell; it undergoes synthesis, processing, transport, and degradation.
When the RNA is isolated from the cells to study, much of the information about their localization in the cell is lost.
Therefore, it is important to detect these microRNA molecules in the cells without disrupting the cells.
DNA and silver nanoclusters can light up RNA
As a nanobiotechnologist, our research team has made use of both our understanding of the biology of DNA and the chemistry of fluorescent nanomaterial to develop a new approach to visualizing RNA molecules.
Our innovative technology exploits unique interactions between DNA and nanomaterials, specifically utilizing ‘Kissing DNA structures’ formed by silver nanoclusters.
‘Kissing DNA’ is a term that refers to a specific structure formed by silver nanoclusters among DNA molecules.
When silver nanoclusters are less than two nanometers in size, they exhibit fluorescent properties, which makes it possible to study the expression level and spatial position of microRNA ‘live’.
To achieve the right size and prevent the silver nanoclusters from growing larger than two nanometers, one needs to use a scaffold. In this case, we use DNA to prepare silver nanoclusters.
When two specially designed strands of DNA come close to each other and form a kind of ‘kissing’ interaction, they form a stable structure with the silver nanoclusters attached to them.
DNA and silver have been put together before, but the specially designed DNA ‘scaffold’ is novel, and allows us to visualize RNA directly in the cell without isolating it.
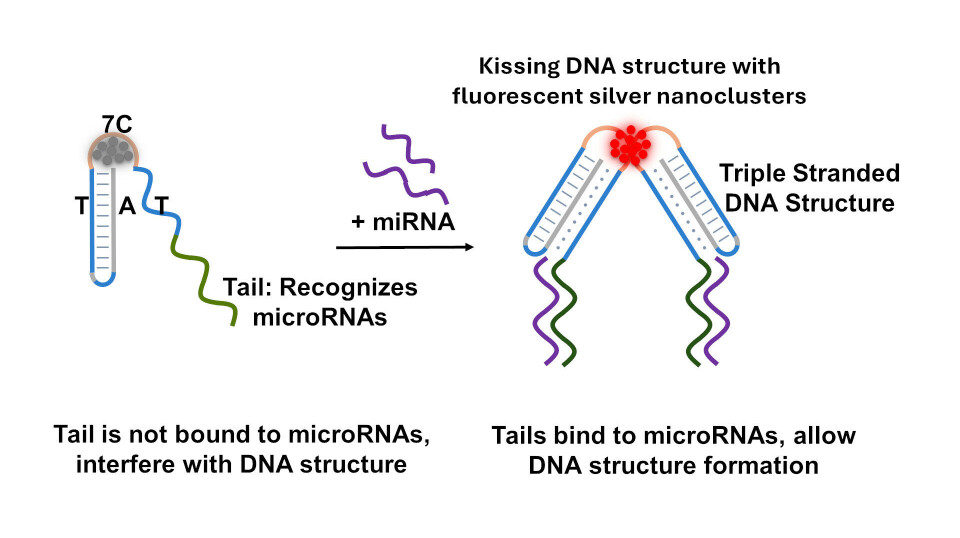
Tailed-Kissing DNA structures
In this technology, we leverage these unique kissing DNA structures to detect microRNAs.
Normally, DNA strands behave like zipper causing two DNA strands to stick together.
Here, we design unusual DNA structures that are present in biology only occasionally and for a very brief period, known as ‘Hoogsteen base-paired triple-stranded DNAs’.
As you might guess from the name, there are not just two, but three strands of DNA in such a structure.
When triple-stranded DNAs are used as a scaffold for silver nanoclusters, they make a kissing-DNA structure that generates bright red fluorescence.
How Kissing DNAs help us see microRNA
In our current research, we made a finding that when we add a tail to the triple-stranded DNA structure, we are interfering with the kissing DNA structure formation.
Think of this tail as an extra piece or extension at the end of our triple-stranded DNA that can recognize microRNAs.
When the tail is occupied by microRNAs, it does not interfere with the kissing DNA structure. This finding created an interesting opportunity to detect microRNA biomarkers for diseases.
Consequently, when a microRNA binds to the DNA tail, the silver nanoclusters can be generated to emit a vibrant red fluorescence, creating a visible signal indicating the presence of a specific microRNA.
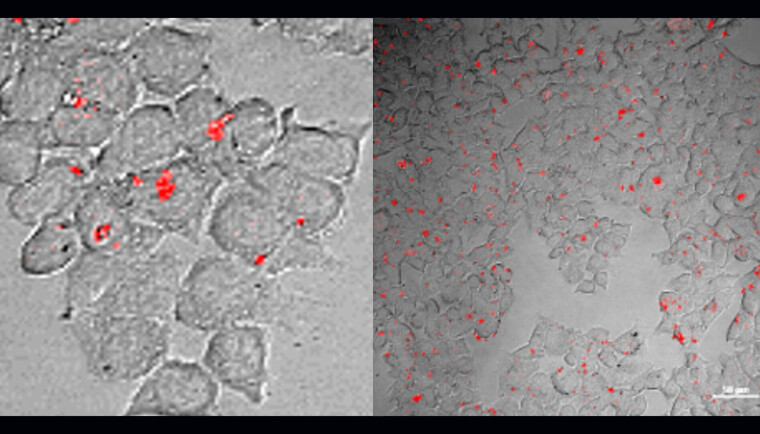
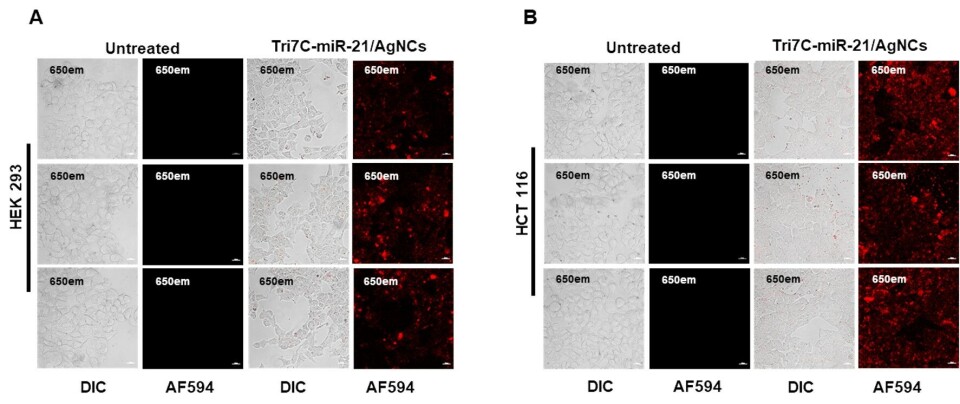
Figure text: The two images show different cell types, HEK 293 and HCT 116, seen through a special microscope. Some of these cells were treated with Tri7C-miR-21/AgNCs (a complex of DNA (Tri7C), microRNA-21 (miR-21) og silvernano-clusters (AgNCs)) for 12 hours, while others were not.
All cells were also treated with lipofectamine, a substance that helps bring molecules into the cells, which attracts microRNA to the kissing DNA structure.
We see red fluorescence from the cells treated with Tri7C-miR-21/AgNCs. In other words, when the cells were treated with Tri7C-miR-21/AgNCs, microRNA-21 lit up in red light, which was captured by the microscope.
Why do we need to learn about the location of microRNAs in cells?
Since the first identification of microRNAs, studies investigating their localization are largely overlooked due to the technical limitations such as the small size of microRNAs.
Here, we have demonstrated that this new technology can be a solution. To truly exploit the diagnostic potential of microRNAs, it is important that we can track the localization of RNA in the cells.
Research (see here, here and here) has demonstrated the localization of microRNAs in organelles such as mitochondria and their potential roles in cardiovascular diseases, Alzheimer’s disease and diabetes.
Here, it remains to be seen if and how the localization of RNA in different organelles within cells changes under healthy and diseased conditions.
We hope our new method can enable such studies without the need to destroying the cells to isolate RNAs.
What we still need to learn
There are more than 2500 microRNAs in humans and identifying the reliable and reproducible microRNAs or a set of microRNAs for diagnostic purposes remains to be achieved.
In most diseases such as cancer and neurodegenerative diseases, the potential microRNA biomarkers are identified for early diagnosis.
However, the major challenges persist in the clinical translation of microRNAs as diagnostic biomarkers due to multiple biological and technological reasons.
On the technology side, current methods used for the detection of isolated microRNAs involve multiple steps, complex reactions, and expensive reagents.
However, since our kissing-DNA structures detect microRNAs in cells, we are keen to translate this know-how to also improve the detection of isolated microRNAs to enable early diagnosis.
A faster and cheaper method
The strength of our technology lies in its simplicity. Compared to current methods, our technology eliminates the need for specialized instruments, expensive reagents, or complex multistep processes.
Moreover, the method that takes hours to detect microRNAs for imaging in cells, can detect isolated microRNAs in just 30 minutes.
We hope to develop this method further using isolated microRNAs from human biofluids or tissues to enable early diagnosis of diseases.
Here, a better understanding of the biology of microRNAs can be useful to know which microRNAs can be reliable biomarkers and their biological role emerging from their visualization in the cells over a lifecycle.
What is next for the DNA silver nanoclusters?
What we have shown here is that DNA silver nanoclusters has the potential to improve our understanding of our biology.
More importantly, we have shown the fluorescent properties of silver nanoclusters can be tuned, based on the structure of the DNA.
This opens new possibilities to expand the DNA design forimaging numerous biomarkers in the cells.
Examples of this include the visualization of reactive oxygen species (ROS) in the cells. ROS are highly reactive molecules that can cause significant damage to cells and tissues.
In the context of diseases, an excess of ROS can lead to oxidative stress, serving as biomarkers for conditions such as cancer, diabetes, and neurodegenerative disorders. Understanding and detecting ROS levels are crucial for early diagnosis and treatment of these diseases.
We are developing DNA silver nanoclusters to enable visualization of these additional biomarkers for a comprehensive understanding of disease development and to develop tools to detect a panel of biomarkers for early disease diagnosis moving forward.
Sources:
- 'Tailed-Hoogsteen Triplex DNA Silver Nanoclusters Emit Red Fluorescence upon Target miRNA Sensing' Nano Micro Small (2015), DOI: 10.1002/smll.202306793
- 'Subcellular Localization of miRNAs and Implications in Cellular Homeostasis' Genes (Basel) (2021), DOI: 10.3390/genes12060856
- 'MitomiRs: new emerging microRNAs in mitochondrial dysfunction and cardiovascular disease' Hypertens Res (2020). DOI:10.1038/s41440-020-0423-3
- 'Mitochondrial MicroRNAs in Aging and Neurodegenerative Diseases' Cells (2020), DOI:10.3390/cells9061345





