Researchers' Zone:

Who came first, sponges or comb jellies?
A Danish biologist has gone through more than 1,000 articles in an attempt to find morphological characters, which could help to settle the debate about which of the two groups forms the most basal branch on the evolutionary tree of the animal kingdom.
For more than a century, there has been almost total consensus among biologists that the sponges form the most basal branch on the animal tree, but new studies on DNA sequences (phylogenomics) are challenging this conception.
Some molecular biologists believe that we should instead look at the so-called comb jellies as our most distant relatives on the animal tree.
This controversy seems to persist, and it has been suggested that studies on morphology (and large molecules) could contribute important information.
This has been the aim of my study, where I have examined more than a 1,000 scientific articles in a search for information about who came first: Sponges or comb jellies?
Existing species shed light on our ancestors
All animals, from sponges and comb jellies to insects, fishes and humans, have particular characters in common: We are multicellular and have sexual reproduction with sperm and eggs (oogamy).
All cells in the animal tissues are diploid (with two sets of chromosomes, 46 in the case of humans), the only exception being the eggs and sperm, which are haploid (with 23 chromosomes in humans); the fertilized egg (the zygote) is diploid.
According to Darwin’s theory of evolution these characters must have been found in the last common ancestor of all animals. We believe that the ancestor lived in the oceans more than 550 million years ago, but we can only guess what it looked like and how it lived.
We have no fossils of these early ancestors, so we have to look at existing species to shed light on the founder of the animal kingdom.
To analyze the basal part of the animal tree, we must include information about the organisms which we believe were closest relatives (sister group) of the animals. The choanoflagellates seem to be the best candidate for this position.
Intracellular digestion in both sponges and choanoflagellates
The choanoflagellates are unicellular flagellates living solitary or in colonies in freshwater and in the sea. Sexual reproduction has only been observed recently.
Each choanoflagellate cell has a ‘collar complex’ consisting of a ring of microvilli (microscopic rod-shaped outgrowths from the cell surface) surrounding an undulating cilium, which propels water along the cell body; bacteria get caught on the outside of the collar and swallowed, so the digestion is intracellular.
Significantly, a very similar structure is found in the feeding structures (choanocyte chambers) of the sponges. Furthermore, almost all animal cell types show structures which can be interpreted as modifications from structures found in a choanoflagellate-like ancestor.
This character complex supports the interpretation of the Animalia (all animals) and the choanoflagellates as descendants from a choanoflagellate-like ancestor.
Interestingly, it has recently been found that fertilization in choanoflagellates is between almost similar cells (isogamy), as opposed to the oogamy of the animals. It further appears that all cells except the zygote are haploid (or there is a short diploid phase), as opposed to the diploidy of the animals.
This character, together with the multicellularity, defines the Animalia as a ‘natural’ (monophyletic) group.
Origin of the animal kingdom
In 1866, Ernst Haeckel (known as Darwin’s apostle in Germany) drew the first phylogenetic tree to visualize his idea of the evolution of the living beings from one common ancestor (figure 1). His tree has subsequently been modified numerous times, the first important modification being that the sponges were included in the Animalia as the most basal branch on the tree.
The animals (Metazoa) are multicellular, and the most important characters of multicellularity is that the cells are so intimately connected that they can share nutrients.
This made it possible for some cells to specialize into new cell types, such as muscle cells and nervous cells, because not all cells have to feed. One theory proposes that the ancestor of the animals was a sphere of choanoflagellate-like cells, which had evolved intimate cell contacts so that they could share nutrients, an ancestor called choanoblastaea (see here, here and here).
The evolution of the basal animal groups from this hypothetical ancestor is now explained in the two competing theories: The sponges (Porifera)-first and the comb jellies (Ctenophora)-first theories.
I have plotted the evolution of morphological characters on the respective evolutionary trees (see figures 2-4).
Three character complexes, which support the Cnidaria + Bilateria branch on the tree, are included in the figures but are not discussed further.
1) The neurotransmitters (molecules, which are used in communication between cells) are completely different in this branch and in the ctenophores.
2) The HOX genes, which are instrumental in patterning of the body plan in bilaterians, are present in the full extent in bilaterians and in a more simple form in cnidarians, but they seem to be completely absent in ctenophores.
3) The presence of a small ciliated sensory organ, the apical organ, at the pole opposite to the blastopore in almost all ciliated larvae, except in the sponges and ctenophores.
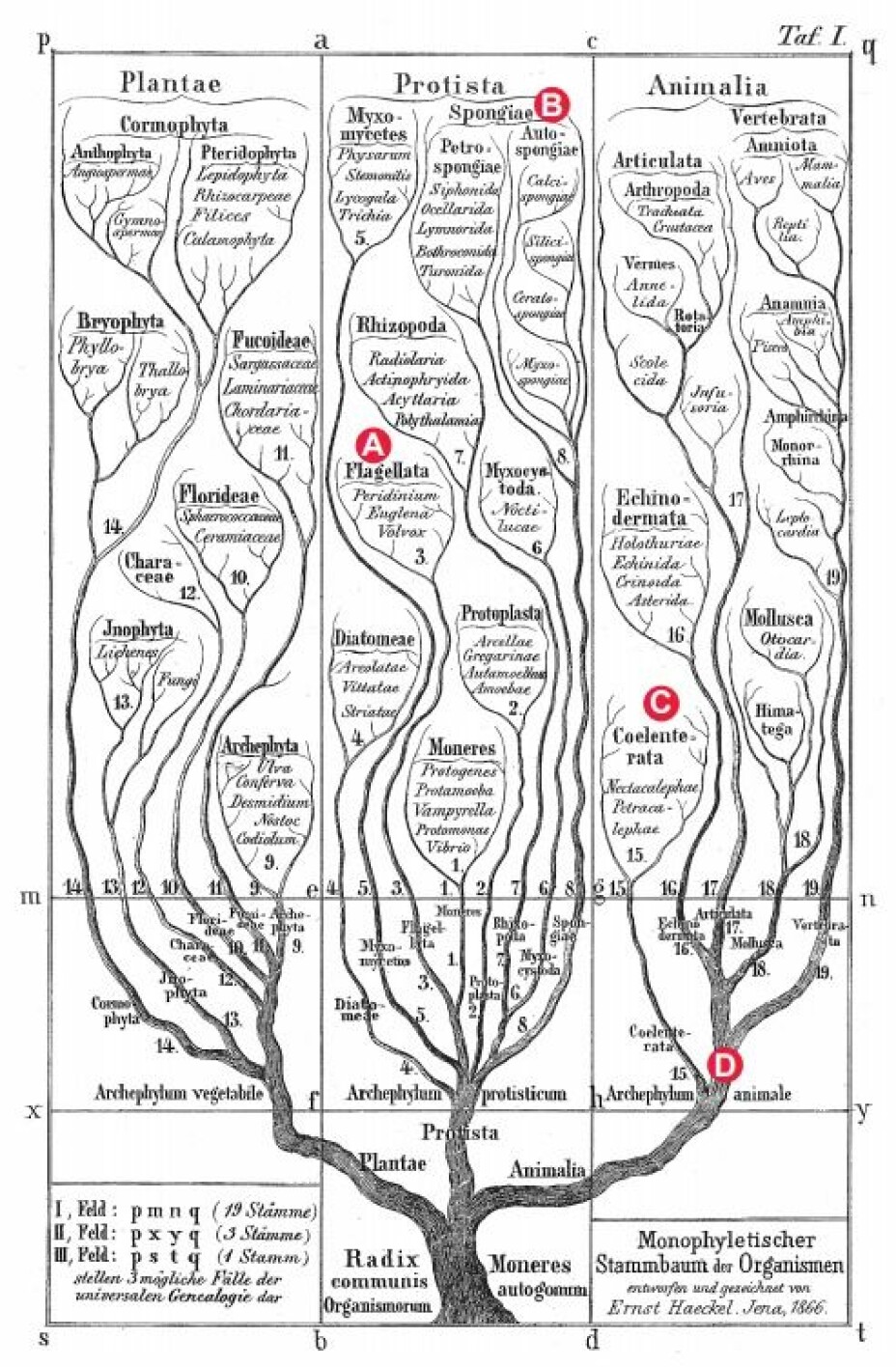
The modern version of both theories interpret the nervous systems of comb jellies and polyps+bilateral animals to have evolved independently, so this character is included in the trees, but is not discussed.
The traditional theory: Sponges and eumetazoans are sister groups
This theory states that the sponges form the most basal branch on the animal tree and has been the consensus view for more than a century. What is the theory based on and what can we say in its defense?
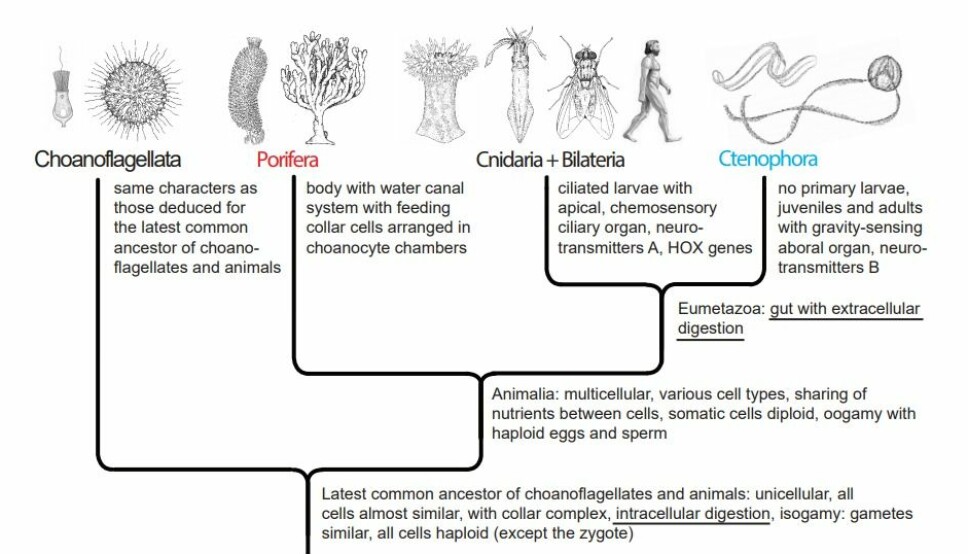
The sponges have collar-cells (choanocytes) of a very similar structure and function as that of the choanoflagellate cells, but arranged in choanocyte-chambers inside the body of the sponges. This indicates, that the feeding with a collar complex and intracellular digestion is a ‘primitive’ life style, which has been retained in the sponges.
The sponges developed a life-style with planktonic larvae and sessile adults. The adults had the choanocytes concentrated in choanocyte chambers connected by water canals together producing a water-current through the sponge for respiration and feeding.
This life style apparently made it impossible to evolve types of free-living adults with ingestion and digestion of larger objects.
The eumetazoans (all animals except the sponges) developed a division of labor between an outer protective cell layer (ectoderm) and an invaginated layer of digesting cells forming a gut (endoderm).
The gut is sac-shaped in the Cnidaria, sack-shaped with a pair of small apical canals ending in anal pores in the Ctenophora, and tubular in the Bilateria.
The gut is a closed space, where the endodermal cells secrete digestive enzymes, which can digest ingested organisms, and from where the cells then take up the digested food, i.e., extracellular digestion, as opposed to the intracellular digestion in choanoflagellates and sponges.
The evolution of a gut made the ingestion of larger organisms possible, and this would have given an important evolutionary advantage. It made it possible to evolve many different life styles, both sessile, creeping and swimming, and with many different feeding types, such as carnivores, sediment eaters and grazers.
As a consequence of the evolution of a gut, the eumetazoans lost the feeding choanocyte cells, because this cell type lost its function in the new gut with extracellular digestion.
But as the ancestral cell type of the animals was choanoflagellate-like, it is not surprising that almost all animal cell types show structures, which can be interpreted as modifications from structures in a choanoflagellate-like ancestor.
This is a strong indication of that the Eumetazoa is a ‘natural’ (monophyletic) group.
The tree in figure 2 shows the traditional picture of basal animal evolution with serial addition of new characters, which give adaptational advantages and no loss of ‘useful’ characters.
The new theory: The comb-jellies are the basal branch on the metazoan tree
Did comb jellies arrive before the sponges? Are they our most distant relatives on the animal three? That is what this new theory is claiming. Let us have a look at this new theory and the evidence behind it.
The comb jelly-first tree (figures 3 and 4) shows a tree with a branch consisting of Porifera + Cnidaria + Bilateria, a branch for which no shared, new morphological character has been identified.
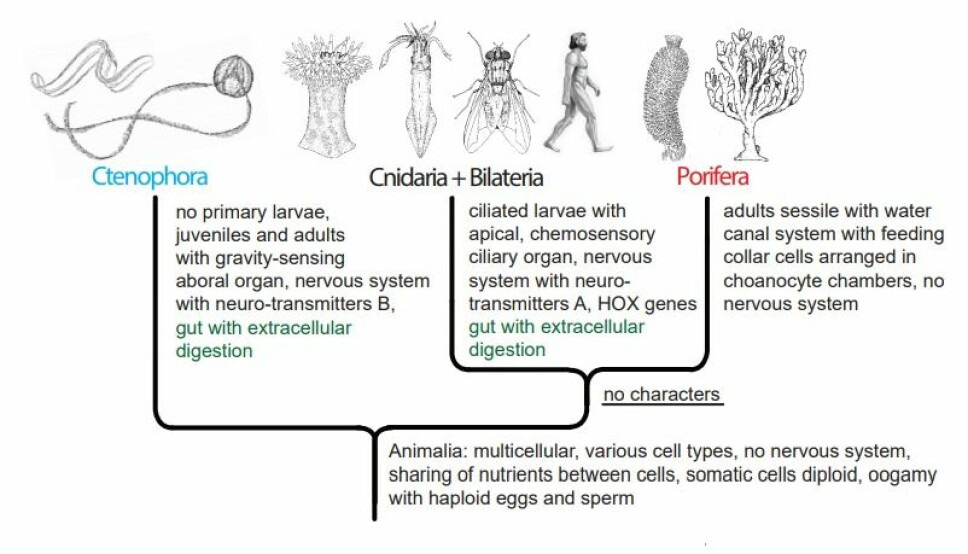
This absence of new characters distinguishing the Porifera + Cnidaria + Bilateria group from the Ctenophora makes this branching at the tree highly improbable, and to my knowledge no name has been proposed for the group.
The theory further implies either independent evolution of the gut with extracellular digestion in Ctenophora and in Cnidaria + Bilateria (alternative A, figure 3) or loss of the gut with extracellular digestion and consequently the ability to digest larger organisms in the Porifera (alternative B, figure 4).
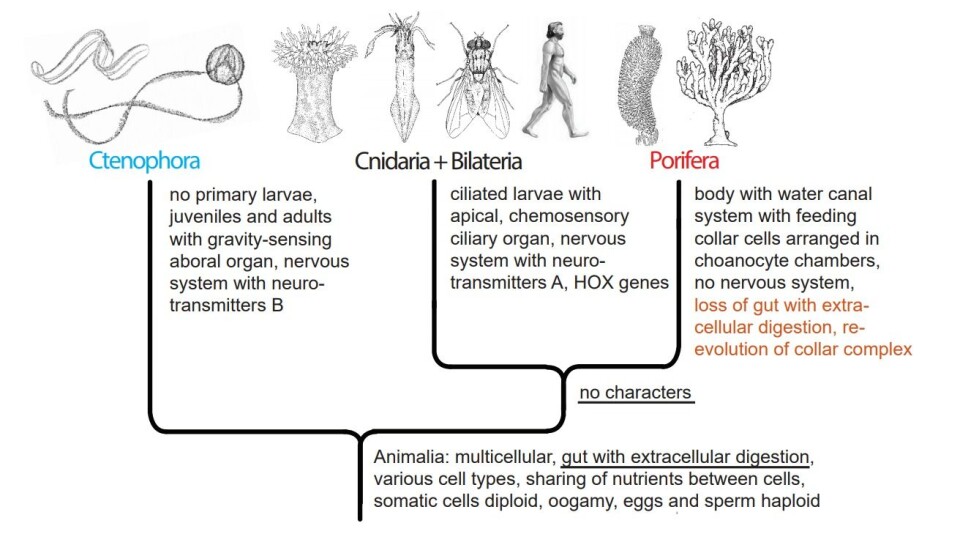
Both alternatives sound improbable, although not impossible.
Important information about gut evolution (alternative A) could be gained by studying the digestive processes in representatives of the two lineages. At present, the digestion in Ctenophora has not been studied at the biochemical and molecular level, and the digestion of the sponges is poorly known.
Alternative B (figure 4) implies that the Porifera should have lost the extracellular digestion in a gut with the endoderm secreting the digestive enzymes, and ‘returned’ to feeding only on bacteria, which are digested intracellularly.
It seems impossible to see any evolutionary advantage of losing the ability of ingesting and digesting larger organisms, and at the same time re-evolving the feeding choanocyte structure and the intracellular digestion.
So, how do the two evolutionary trees compare?
The Porifera-first tree (i.e. sponges came first) shows uninterrupted stepwise addition of new characters, and loss in the eumetazoans of the choanocytes, which had lost their function with the evolution of the gut.
This is in complete agreement with our traditional thinking about evolution through addition of new characters, which give an adaptational advantage, and loss of characters, which become redundant.
The Ctenophora-first theory (i.e., comb jellies came first) firstly implies an evolutionary branch for which no new character has been identified. This is not in accordance with traditional logic.
Secondly, it implies either important parallel evolution of gut with extracellular digestion in the two eumetazoan groups Ctenophora and Cnidaria + Bilateria, or the loss of this important character in the Porifera. Both alternatives appear highly improbable.
We can thus conclude that morphology gives strong support to the classical Porifera-first theory, saying that the sponges were the first side branch on the animal evolutionary tree.
Read the Danish version at Videnskab.dk's Forskerzonen.
References:
'Generelle Morphologie der Organismen. 2 vols', Georg Reimer (1866)
'Evidence for sex and recombination in the choanoflagellate Salpingoeca rosetta', Curr. Biol. (2013), DOI: 10.1016/j.cub.2013.08.061
'Animal Evolution: Interrelationships of the Living Phyla', Oxford University Press (2012)
———






