Learning from bacteriophages to develop targeted antimicrobials
Scientists are learning from nature to develop new ways to combat antibiotic resistant bacteria and boost food safety.
Set a virus to kill bacteria, or even better, learn from viruses to kill bacteria.
As antibiotic resistant bacteria continue to haunt the globe, many scientists have shifted their focus to find alternative solutions to this emerging problem.
One promising alternative is the use of bacteriophages, known simply as phages—the natural born killers of bacteria. Upon binding to a susceptible bacterium, these infectious viruses inject their genomic material into the bacterial host and hijack their metabolism for phage replication, which ultimately kills the bacterial cell.
So far, research on these viruses has led to the discovery of numerous tools that are highly beneficial in the war against bacteria. But scientists are now taking it to the next stage: combining the selectivity of phages in binding to a specific bacterium with potent nano-injectors to produce efficient and specific antimicrobials. These will be capable of killing the most prevalent food-borne pathogens, Campylobacter jejuni and Salmonella.
This will eventually help us to reduce the presence of dangerous pathogens in foods, and consequently improve public health and decrease the deaths and the economical burden resulting from food poisoning.
Read More: Scientists fling bacteria’s “black box” wide open
Phages kill specific bacteria
Being the most abundant entities on the globe, phages are very diverse yet highly specialised viruses that only kill bacteria.
Approximately 95 per cent of all phages described today have “tails” comprised of complex protein structures. These tails can be short or long, contractile or non-contractile and quiet flexible or very rigid.
The protrusions found at the end of the tails are either spikes that resemble short nails or fibres that resemble spider legs and function as the initial point of contact with the bacteria. It is these protrusions that determine suitable bacteria for the phage to infect and consequently determine whether or not the bacteria will be killed.
As a result, these tail structures have evolved for thousands of years with one crucial purpose; to recognise the suitable bacteria in the vast bacterial cosmos. The tail structures do this by binding to a specific receptor component that is found on the surface of the bacteria such as sugars or proteins.
While some phages bind to receptors that are commonly found and conserved in many bacteria, others carry more specialised versions of these tail structures that recognise a specific surface component that is unique to a certain bacterium. These tail structures are also known as the receptor binding proteins (RBPs) of phages and these very specialised proteins are an attractive tool to target specific groups of bacteria.
Read More: Statistics might save us from resistant bacteria
Nano-injectors kill bacteria
Scientists know that some bacteria produce nano-injectors that kills similar or closely related bacteria that are competing for the resources in the same environment. Such nano-injectors are highly ordered complex proteins that look similar to phage tails and also carry tail fibres that bind to a specific receptor on the target bacteria.
This nano-injector kills the target bacteria by binding to the specific receptor and then punctures the bacterial cell by injecting a hollow tube. Such nano-injectors are highly potent, as one particle is enough to kill a single susceptible bacterial cell.
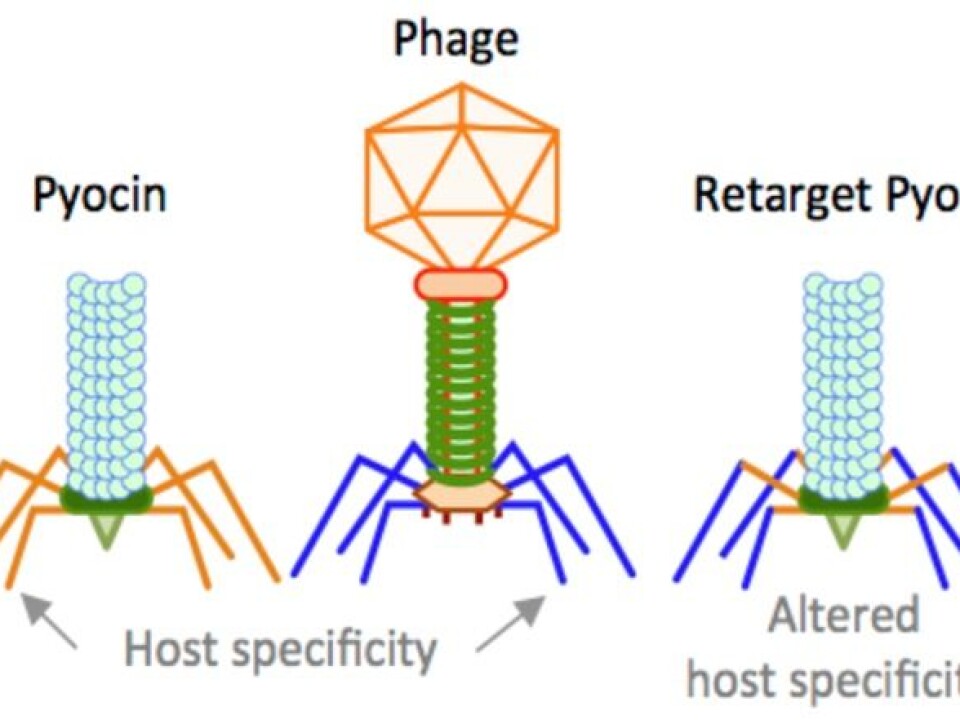
One such type of a nano-injector is the so-called R-type pyocin produced by the bacteria, Pseudomonas aeruginosa.
Genes that make up this type of nano-injector are similar to phages with contractile tails. Therefore, by exchanging the tail fibres of pyocins with different phage RBPs, we are re-targeting this nano-injector to kill bacteria other than Pseudomonas.
Read More: Gut bacteria will revolutionise medicine
Killing Campylobacter and Salmonella to increase food safety
In our research group, PhageBio at the University of Copenhagen, we have a large collection of phages that are able to kill food-borne pathogenic bacteria.
Our first goal is to primarily target C. jejuni and Salmonella as they are the most commonly implicated agents of food poisoning in humans in Denmark and elsewhere in EU. These two bacterial species are often found in farm animals with no symptoms and contaminate the food throughout the production chain, and ultimately cause infections in humans.
For most of the phages in our collection, we know the receptors they target and the bacterial host ranges they posses. In our on-going research project, we will exchange the tail fibre tips of pyocins with RBPs from our phages, thereby using the specificity of the RBPs and the potency of nano-injectors to kill Campylobacter and Salmonella.
In this approach, the bacteria will simply be killed when the re-targeted pyocin binds to it.
Our approach also allows us to study and discover novel RBPs from phages that kill the worst bugs—the multi-drug resistant bacteria. Ultimately, we will be able to apply this approach to develop novel antimicrobials against virtually any bacteria, as long as we know the phages infecting the bacteria in question.
Read More: Why we should use these bacteria in fertilizers
Future work will target bacteria at the farm level
Currently, we are at the stage of investigating the re-targeting capability of some of the RBPs originating from C. jejuni and Salmonella phages. This step is very exciting, as it will show the killing capacity of these re-targeted nano-injectors.
The next step will be to see how efficiently they can kill pathogens in food, without affecting sensory or microbiological quality of the food.
And finally, we will see how efficiently these nano-injectors will control specific pathogens in live animals at the farm level. For example, they could be administered via animal feed or water, to clear the pathogenic bacteria from the guts without affecting the beneficial gut microflora.
---------------
Read this article in Danish on Videnskab.dk
This project is funded by European Union’s Horizon 2020, under Marie Skladowska Curie Individual fellowship programme.