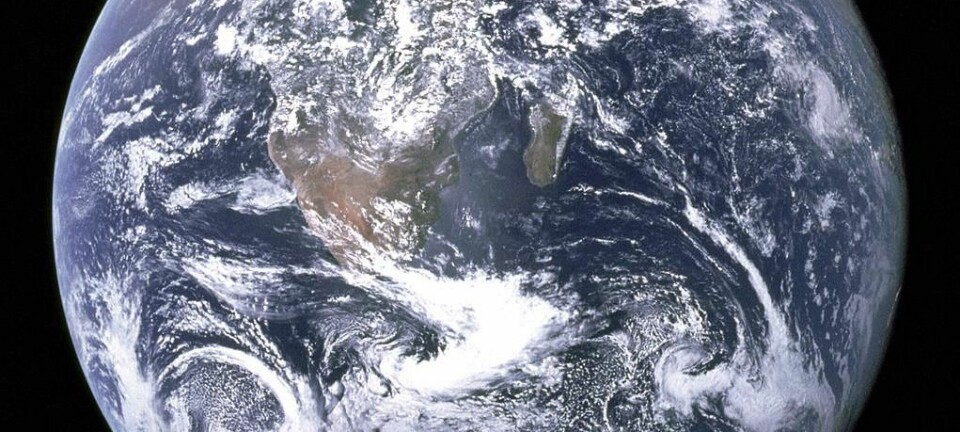
The Earth has lost a quarter of its water
In its early history, the Earth's oceans contained significantly more water than they do today. A new study indicates that hydrogen from split water molecules has escaped into space.
Although water covers 70 percent of the Earth's surface, water is actually a rare substance that represents just 0.05 percent of the Earth's total mass.
Water has nevertheless played a crucial role in the emergence of life on Earth. Without water, the Earth would in all likelihood be a dead planet.
The amount of water on the planet has not always been the same, however. A research group at the Natural History Museum of Denmark has discovered this by measuring how hydrogen isotope ratios in the oceans have changed over time.
"The water that covered the Earth at the dawn of time contained more of the lighter hydrogen isotope than the heavier hydrogen isotope, known as deuterium, than it does today,” says Emily Pope, a post doc, who has played a central role in the study.
“By examining how the ratio of these isotopes has changed, we have been able to determine that over the course of around four billion years, the Earth's oceans have lost about a quarter of their original mass."
Geological clues in Greenland
Pope and her colleagues found their path to discovery in a mineral called serpentine.
Serpentine is formed when the Earth's crust comes into contact with seawater circulating at high temperature through channels and cracks in the Earth's crust beneath the seabed.
The isotope ratios in serpentine are determined by the isotope ratios in the sea water at the time the mineral was formed, and this information can be used to form a picture of what the oceans were like aeons ago.

Serpentine is a relatively commonly occurring mineral, but the researchers chose to look in the Isua Belt in western Greenland where some of the Earth's oldest rocks were formed, 3.8 billion years ago.
In 2010, Emily Pope, together with colleagues Minik Rosing and Dennis K. Bird went to a part of Isua, previously identified as being an ancient seabed rich in serpentine, to collect samples.
Hydrogen floats off into space
Rock samples were taken from the area and subsequently analysed in a laboratory at Stanford University in California, USA.
Tests revealed a significantly higher ratio of hydrogen to deuterium than is seen today.
The explanation, according to Emily Pope, is that when the Earth was in its infancy, part of the water in the oceans was split into hydrogen, deuterium and oxygen via a process called methanogenesis. Both hydrogen and deuterium are low-density gases, so they rose through the atmosphere and eventually floated off into space.
Methanogenesis works more efficiently for hydrogen than for deuterium, so more hydrogen gas was created by this process than deuterium gas, and this slowly but surely altered the ratio of these isotopes in the oceans.
Knowing how much hydrogen had disappeared from the oceans over the last four billion years enabled the researchers to calculate that the oceans have lost about a quarter of their water since the Earth’s early days.
"Hydrogen and deuterium are still escaping into space, but very slowly, says Pope.
“Today the atmosphere is rich in oxygen, which reacts with both hydrogen and deuterium to recreate water, which falls back to the Earth's surface. So the vast bulk of the water on Earth is held in a closed system that prevents the planet from gradually drying out."
Young Sun Paradox
The analyses also showed how much methane existed in the atmosphere of the infant Earth. The methanogenesis process creates hydrogen from methane, and since the researchers know how much hydrogen was lost to space, they were also able to estimate how much methane the atmosphere must have contained in the past.
Their calculations show that at the time when the rocks in the Isua Belt in Greenland were formed, the atmosphere contained 50 to 500 times more methane than it does today.
This result is relevant to the debate on why the Earth's climate in prehistory was almost as warm as it is today, despite the fact that the Sun was significantly fainter – an apparent contradiction known to researchers as the Young Sun Paradox.
One solution to the paradox is that the atmosphere at that stage in the Earth's history contained large amounts of greenhouse gases.
But this hypothesis is invalidated by the study from Pope and her colleagues.
"We have found that the atmosphere contained more methane than it does today,” she says. “But it was still a fraction of the amount necessary to create a warm climate solely using atmospheric methane as a greenhouse gas."
Dramatic climate change
The reason for the climate being so warm must have been something other than atmospheric greenhouse gases. Pope favours a theory proposed by Minik Rosing and others in 2010.
Their explanation of why the climate was warm despite a fainter sun is that the surface of the Earth was covered with water at that time, whereas today the Earth's surface is partly land mass.
Seawater absorbs more sunlight than land does, so a larger amount of energy was absorbed when oceans covered the planet. It is argued that this larger energy absorption was sufficient to keep the climate relatively warm.
Minik Rosing, who also participated in the new study, emphasises that the new results not only reveal something about the past climate, but also put current climate change in perspective.
"The Earth's climate has so far been a stable system. Current climate change, for which the human race bears much of the responsibility, is dramatic compared to the small variations that have taken place over time,” says Rosing.
“When we increase the amounts of greenhouse gases in this way, an imbalance results which perhaps can never be re-stabilised – a balance that has been the reason why life was able to come into being and flourish."
-----------------------------------
Read this article in Danish at videnskab.dk
Translated by: Nigel Mander