Why leprosy is still going strong
Scientists have mapped the genome of the bacterium that causes leprosy. The findings reveal why the disease still manages to infect more than 200,000 people every year.
Leprosy has plagued us humans for thousands of years.
In medieval Europe, the disease was so dreaded that lepers had to wear a bell around their necks to warn others of their condition.
But that doesn’t mean leprosy is a thing of the past. It still manages to infect more than 200,000 people a year.
This makes the leprosy bacterium Mycobacterium leprae one of the most persistent infectious bacteria in human history.
Practically all Danes in the 13th and the 14th centuries were infected with leprosy. But since the disease don’t break out until later in life, many people died of other causes before the disease broke out.
Jesper Lier Boldsen
And now an international research team has figured out the secret behind this staying power:
“The genome sequencing of the Mycobacterium leprae revealed that the bacterium hasn’t changed significantly for at least 1,000 years. This is a bacterium which for a long time has been perfectly adapted to life inside us humans,” says the Danish contributor to the study, Research Professor Jesper Lier Boldsen of the Institute of Forensic Medicine at the University of Southern Denmark.
The study can help us better understand the disease today and in the past.
DNA from 1,000-year-old bacteria
The researchers extracted bacterial DNA from the skeletons of five people who died from leprosy in England, Sweden and Denmark between the 10th and the 14th centuries.
This DNA was sequenced and then compared with the genome of today’s Mycobacterium leprae.
The results showed that the bacterium has changed very little over the centuries. And there’s a reason for that:
“If there was an advantage in evolving further, it probably would have done so,” says Boldsen. “But it hasn’t. It has found itself a niche where it has managed to coexist with humans for a long time and with a widespread presence.”
The trick lies in the long incubation period
The long-running ‘success’ of leprosy can be explained with reference to the course of the disease.
Most lepers are infected with the disease in their childhood, but the disease doesn’t break out until later in life.
A previous study of a large epidemic in Norway, lasting from 1850 to 1920, showed that for half of those infected, the disease didn’t break out until after they had turned 35.
”So it is a bacterium that can live in humans for many years without killing them. It manages to infect a great number of people before the first signs of the disease are noticed,” he explains.
“One consequence of this is that practically all Danes in the 13th and the 14th centuries were infected with leprosy. But since the disease doesn’t break out until later in life, many people died of other causes before the disease broke out.”
Indian lepers excluded
Today, the majority of those infected live in India. Here, many of them live in modern versions of medieval leprosy colonies.
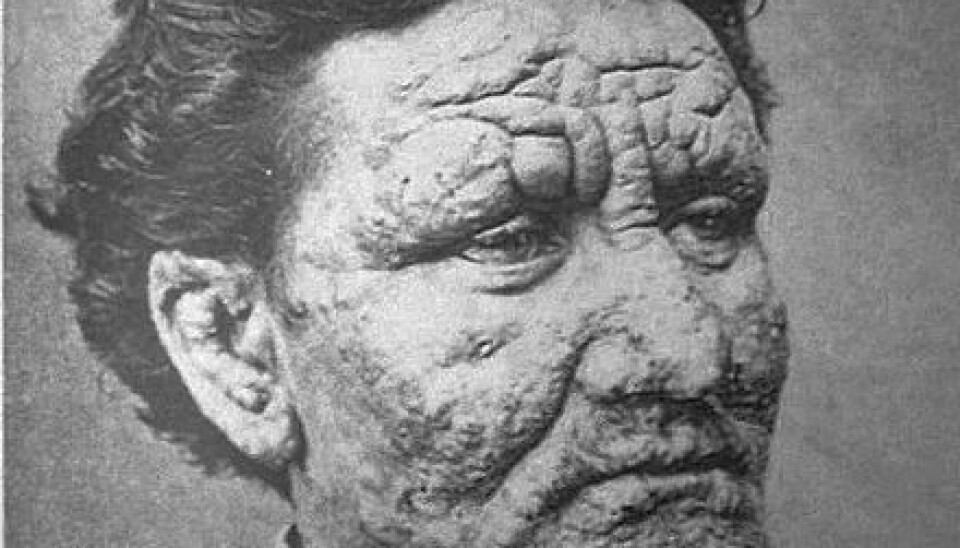
The visual signs of the disease, which include severe skin rash on the face, make it one of the most stigmatised diseases of all time. The same is true today, where Indian lepers are often excluded from the local community and are forced to stay at one of the country’s more than 1,000 leprosy colonies.
Study boosts our understanding of leprosy
The new study provides unique historical insight into a disease that has followed humans for centuries. And the genome sequencing of the Mycobacterium leprae may also be of practical use:
“This is valuable general knowledge about an infectious bacterium that we can use in the further development of treatments and prevention. Today, leprosy remains one of the main causes of acquired blindness in the world,” says the researcher.
“With a better understanding of the bacterium’s genome, we may be able to reduce the number of infected people.”
----------------------
Read the Danish version of this article at videnskab.dk
Translated by: Dann Vinther