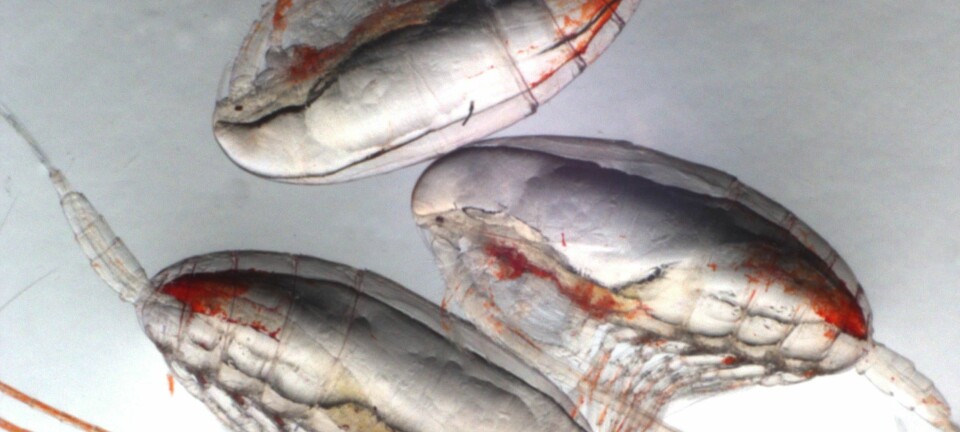
Ocean acidification might not spell doom for shellfish
New study claims that ocean acidification may not have such alarming effects on calcified marine animals as many scientists have predicted.
The ocean is slowly soaking up more and more of the CO2 that we have been pumping into the atmosphere since the start of the industrial revolution. And as a result it is becoming more acidic.
This process, known as ocean acidification, poses serious problems for many marine animals made of calcium carbonates like clams, cockles, mussels, and sea urchins. Experiments show that as the ocean acidifies, these animals might not be able to grow as much as they do today, or maintain their calcium carbonate shells.
Read more: Top four ocean threats according to marine scientists.
But a new study suggests that a more acidic ocean might not have such dire consequences for these calcified animals as many scientists predict, including the UN’s Intergovernmental Panel on Climate Change (IPCC).
The secret, they say, is food.
“Food gives energy to the organisms, which they need to cope with the problems of low pH,” says co-author Dorte Krause-Jensen from Aarhus University, Denmark.
“So as long as they are fed they can actually grow and calcify almost as if conditions were normal,” she says.
The new results are published in the journal Nature Scientific Reports.
Animals need more energy to cope with ocean acidification
Just as top athletes need to consume more calories to sustain their level of physical activity, these animals need food to cope with the stresses of living in a more acidic ocean.
But many of the existing studies into the effects of ocean acidification are based on experiments in which the animals were not fed. And this may have significantly biased their results, says Krause-Jensen.
So she and her co-authors looked closely at the results of these experiments to see what effect food supply had on the animals exposed to more acidic water.
“These experiments showed that if the organisms are not fed and exposed to reduced pH, then they have problems calcifying and their growth is also reduced,” says Krause-Jensen. “But if the same organisms have food while exposed to these reduced pH conditions, then they are able to cope with it.”
The results may even call into question some of the conclusions of the IPCC report concerning the negative impacts of ocean acidification.
“The results that are used in the IPCC are built on studies where half of the experiments took place using organisms that weren’t fed. So there’s a risk that these experiments could overestimate the effect of acidification on these calcifiers,” she says.
Food is not the only factor
Professor Daniela Schmidt from the School of Earth Sciences, University of Bristol, UK, is a co-author on the IPCC chapter, whose conclusions are called into question by the new study. She does not dispute the basic findings of the new research, but describes the conclusions as “too simplistic.”
“The basic finding that food is important to take into consideration is absolutely right, but just combining food and carbonate chemistry as they do here, doesn’t capture the whole story,” says Schmidt.
She highlights the many other factors that are putting these marine creatures under stress and contributing to reduced growth and calcification.
“CO2 doesn’t just affect ocean chemistry, it’s also causing ocean warming. If the earth gets warmer then an organism needs more food to sustain the same function,” says Schmidt. “In fact, for every 10 degrees of warming, metabolic energy doubles. This means that food intake needs to be much much higher to support the same functions.”
Future food supplies are uncertain
At the same time, future food availability is highly uncertain for these marine animals, and is predicted to vary substantially around the world. They mainly feed off microorganisms in the ocean, which are predicted to become more abundant at higher latitudes as sea ice melts and the oceans warm.
But in the tropics, these types of food supplies are projected to reduce, says Schmidt. “This is where we have coral reefs, which are already affected by ocean acidification and warming. And they will have even less food to sustain themselves.”
Karin Kvale, from the Helmoltz Centre for Ocean Research at Kiel University, Germany, studies marine ecosystems using computer models. She is impressed with the new study, but agrees with Schmidt.
“Thinking at the global scale, many of the biogeochemical models in use predict global declines in primary production [food supplies] over this century, in which case calcifiers [clams, etc.] could face increased stress from declining food availability. This would increase their sensitivity to ocean acidification,” says Kvale.
But she adds that there are also computer models that predict increasing food supplies, and so the exact nature of future food availability for these animals is still uncertain.
Marine biologist, Professor Andre Visser from National Institute of Aquatic Resources, Technical University of Denmark, expands on this. He points out that some of the organisms that these calcified animals eat, are themselves, calcified. And so they too are vulnerable to ocean acidification.
“Ocean acidification has effects right across the ecosystem--and the food supply that allows the animals of this study to resist may very well also be compromised by pH stress,” he says.
How low can pH go?
Krause-Jensen and co-author Mikael Sejr, from Aarhus University, agree with this critique.
“I think that this is also the point that we’re trying to make in our study,” says Sejr.
“[Scientists] have been really focussed on studying pH, and our study underlines that we need to include other factors. Food is one of the key factors but of course there are many others that should be included,” he says.
One such factor is to find out exactly what range of pH these animals already experience as part of their everyday life.
“How often is a mussel experiencing low pH in nature?” asks Sejr.
“We’re talking about a change in pH of about 0.2 to 0.3 units by the end of the century. But if that mussel is already experiencing this kind of variation everyday of it’s life, then this is important information. And these kinds of observational studies are really missing,” he says.
Scientific links
- Food supply confers calcifiers resistance to ocean acidification. DOI 10.1038/srep19374
- IPCC Working Group II report. “Climate Change 2014: Impacts, Adaptation, and Vulnerability.” Go to page 129 for sections related to ocean acidification in the section entitled ‘Cross-chapter box compendium.’