New theory explains how metals melt and freeze
Physicists have discovered how the melting process works at extreme pressures such as those found inside the Earth’s core.
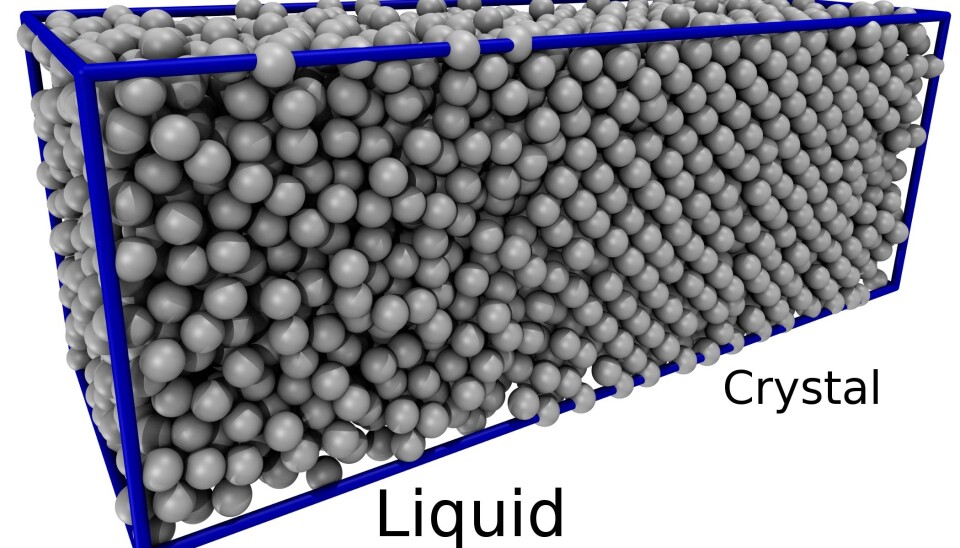
Scientists have developed a new theory of freezing and melting of metals such as iron or copper.
The new results are published online, in the scientific journal Nature Communications.
The scientists behind the new study hope that the theory will bring them closer to understanding how metals develop under the extreme pressure inside the Earth and how the fluid metals solidify as they lose heat to their surroundings and cool.
Melting point increases with pressure
The new results show how the temperature at which a substance melts--the melting point--changes at higher pressure.
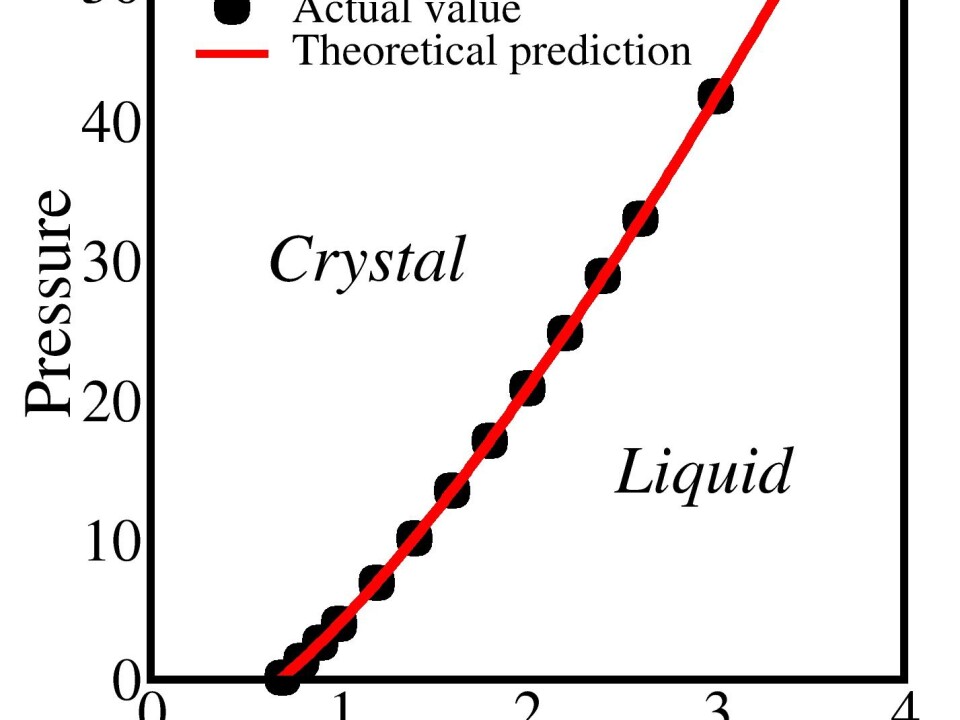
“The melting temperature typically increases when we increase the pressure. For example iron melts at 1,538 degrees centigrade at one atmospheres pressure. But at the high pressure of the Earth’s core, iron first melts at more than 5,000 degrees,” says lead author Ulf Rørbæk Pedersen, from Roskilde University, Denmark.
“The questions is, how melting and freezing phenomena changes as the pressure increases. An interesting suggestion is Lindemann’s theory from 1910,” says Pedersen.
When you heat up a crystal, the molecules start to move around as they vibrate around their positions within the crystal. Lindemann suggests that at some point the vibrations become so violent that the crystal simply breaks down and melts.
“Now for the first time, we can understand how big the vibrations need to be before the crystal melts and that depends on the pressure, which is the opposite of what Lindemann thought,” says Pedersen.
The physicists can now predict how quickly the fluid melts when the melting point is reached and conversely, how quickly the atoms organise themselves when the substance begins to crystallise.
Read More: Breakthrough in physics may lead to new view of magnetism
Insights into how long exoplanets retain their heat
Physicists can now begin to answer key questions such as: Why does a metal melt and freeze at a particular pressure? They can then calculate how this process changes under different conditions, for example, at extreme pressures.
“I hope that our theory can be used to model the extreme conditions, that are found in the centre of the Earth and other planets, including exoplanets,” says Pedersen.
“The theory can tell us how quickly the inner crystals grow, which makes it easier to calculate how long it takes for the planet to cool down,” he says.
The new results could indicate how long exoplanets--planets that orbit stars other than the Sun--can stay warm long enough to support fluid water at their surface, and therefore life.
Read More: New discovery: small planets have circular orbits
-------------
Read the Danish version of this article on Videnskab.dk
Translated by: Catherine Jex