Lack of oxygen led to first mass extinction
The first mass extinction of animal life on Earth was previously blamed on a rise in the oxygen concentration in the oceans as a result of a cooler climate. But a new study shows the catastrophe was really caused by a massive decrease in oxygen.
With its warm climate and plenty of food, the Earth was a good place to live for the many animals with shells, legs and teeth.
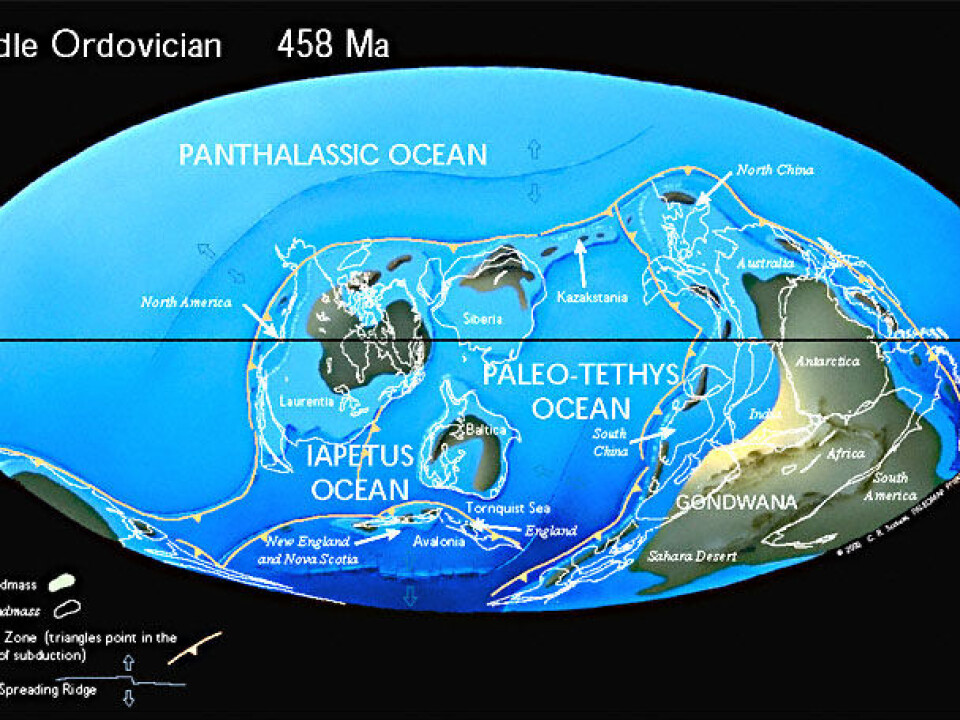
Animal life had just conquered the oceans 440 million years ago. But suddenly something happened that over the following half a million years wiped out almost 86 percent of all species of animals that lived in the oceans.
Researchers have speculated for decades about the cause of this catastrophe, which occurred towards the end of the Ordovician geological period.
To date, the prevalent theory has been that the disappearance of the many species was due to a cooler climate that led to a more effective mixing of atmospheric air in the sea water and thus in an increase in the oxygen concentration in the oceans (See Factbox).
For the first time, however, another clear picture of events is taking shape.
New measurements dismiss previous theories
Emma Hammarlund, a biologist and geochemist at NordCEE, the Nordic Center for Earth Evolution, has closely studied the chemical composition of the late Ordovician seabed.
Surprisingly, her results indicate that the cooler climate is much more likely to have resulted in a reduction in the oxygen concentration in the oceans, and that this was the development that killed off a lot of the animal life.
Her results have just been published in Earth and Planetary Science Letters.
“Our measurements tell us that there was a widespread reduction in the oxygen concentration in the oceans – and not an increase – and this was the cause of the first mass extinction of animal life on the Earth,” says Hammarlund. “These measurements have overturned the old theories about what happened then.”
Old seabed still exists
The catastrophe happened so long ago that the animal life has completely recovered.
However, the Earth has not forgotten what happened millions of years ago, and many of the catastrophe’s most important events have left their mark on the seabed of that time; since then, the seabed has been pressed together to form beds of schist.
The old seabed is no longer intact but has been broken into smaller pieces that have been moved around by the tectonic plates that form the Earth’s surface and by other geological processes.
Hammarlund and her colleagues have taken samples of the old seabed in recent years from sites in Scotland, Austria and the Danish island Bornholm.
By analysing the many samples in their laboratories, the researchers have been able to extract secrets hidden in the schist, thereby getting a far better understanding of the worldwide catastrophe.
The schist contains many metals, sulphur, carbon and other substances that each tell a story about the catastrophe. A precise determination of the chemical composition of the schist gave the researchers a great deal of information about the first mass extinction of animal life.
Sulphur-consuming microorganisms
The element that helped the researchers most in discovering what really happened was sulphur, most of which is found in two different variants (isotopes) – one light, the other heavy.
Hammarlund’s analyses show that the seabed 440 million years ago stored sulphur compounds that were considerably heavier than they had been before.
“That the old seabed was rich in heavy sulphur compounds can in my opinion only be explained in one way: there was a considerable consumption of sulphur at that time due to sulphur-loving microorganisms that could only exist in oxygen-free environments,” she says.
“The heavier sulphur compounds can thus be evidence that the oxygen in the oceans suddenly disappeared and gave the sulphur-consuming bacteria optimal life conditions.”
Lack of oxygen ‘logical explanation’ for mass extinction
Hammarlund believes that the theory about a lack of oxygen is logical in the light of the knowledge we have of the climate then and what happened on Earth in the period prior to the catastrophe.
The catastrophe lasted for about one million years – and earlier studies showed that it occurred in two phases:
- Free-swimming animals and animals living in deeper water died out 440 million years ago.
- The surviving species, which predominantly lived in shallow water, died out half a million years later.
These two dramatic events occurred at the same time as two great changes in the oceans’ mean water level. During the catastrophe’s first phase, the water level fell by up to 100 m, while it rose by the same amount during the second phase.
The fall in water level brought about widespread reduction in the oxygen concentration in the oceans, and this killed many deep-water aquatic animals.
When the water level rose again, the water – deficient in oxygen – swept in over low-water areas, suffocating all the aquatic animals that survived the first phase.
Dramatic fall in water level led to widespread lack of oxygen
Using calculations carried out by Christian Bjerrum, a geologist at the Geological Survey of Denmark and Greenland (GEUS), Hammarlund explains the process in which the fall in water level leads to a widespread fall in oxygen concentration: the oceans receive nutrients from coastal areas, and these release phosphorus, iron and other substances – which is the reason why so many aquatic animals prefer living in coastal areas rather than in deep-water areas.
Dead animals and plants quickly fall to the seabed, where they are buried as new layers of organic material. Along the coast, the distance from the surface of the sea to the seabed is so short that the bacteria in the water cannot consume everything that dies.
When the water level fell by 100 m, what was once open sea became coastal areas. And where the water level gradually became deeper away from the coast, it was now quite deep along the coast.
Algae consumed the nutrients that ran off the coast into the oceans, but now it took far longer for dead algae to sink to the seabed. This meant that the microorganisms could consume most of the algae. As a result, the nutrients were released quickly and could be used again, which led to new algae populations.
But the new biomass also died, so the seabed was covered by very large quantities of dead algae. The process of putrefaction needed large quantities of oxygen, and this was supplied by the water – resulting in a fall of oxygen concentration in the water.
---------------------------
Read this article in Danish at videnskab.dk
Translated by: Michael de Laine