
How spiders make their silk
Spider silk is one of the strongest materials known, and the arachnids make it faster than greased lightning.
Spider silk is stronger than steel and is highly elastic and flexible.
The silk can withstand extreme changes in temperatures, from -40° C to 200° C. Some types of the silk are tougher than Kevlar, the material used in bullet-proof vests.
“It’s astounding that spiders have developed a way of producing the world’s strongest fibre, nearly instantaneously ― and of course it is biodegradable too,” write Anna Rising and Jan Johansson to forskning.no.
This material can be put to multiple uses and a single spider spins several types of silk, including draglines from which to hang their webs and wrapping material for packing in their prey.
So how does this natural but super substance get made?
It starts in the ampulate gland
Swedish, Chinese and American scientists have joined forces to find out how the spider silk synthesizing process is regulated.
The silk consists of so-called spidroins, large proteins which are created in the ampulate gland. When a spider wants to make silk, it converts these proteins into a solid fibre in a tiny fraction of a second.
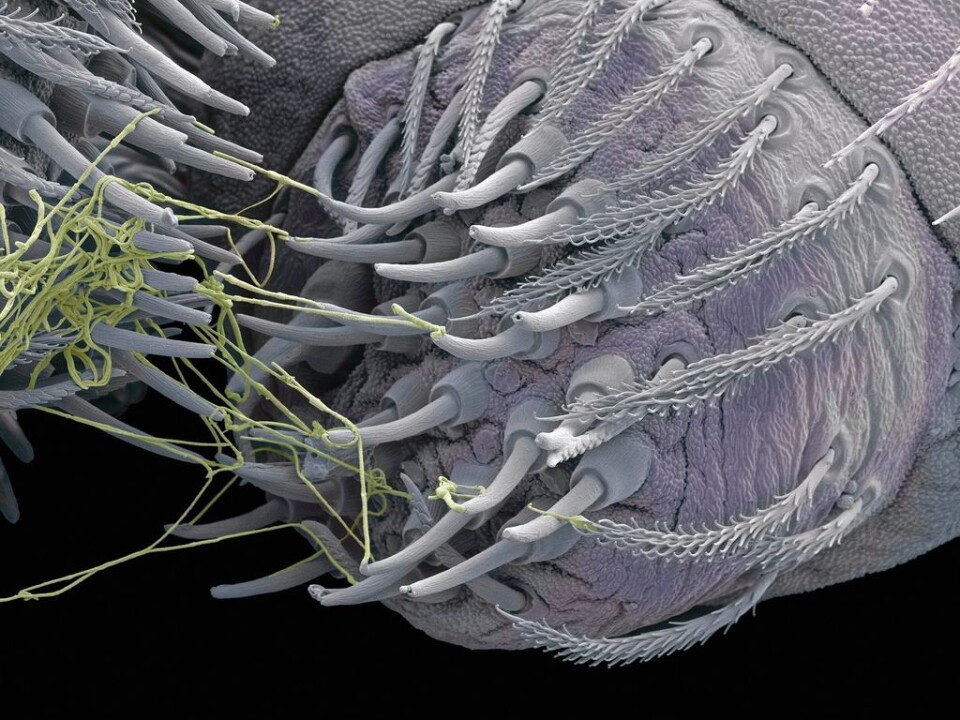
Most spiders have many of these glands, each making a single fibre. These are spun together to spider silk in the organ known as its spinneret.
While in the ampulate gland, the spidroins are stored in a gel state prior to use.
The gland narrows down into a duct where it is turned into silk fibre.
Water is removed from the fibre and at the end is a spigot which regulates the diameter of the silk fibres and spins the silk.
Lower pH value and converted proteins
It has been known for some time that the silk is made in a particular part of these ducts, but scientists have not fully understood what happens with the spidroins during this process. These proteins have a disorganised structure while in a highly concentrated gel state and dissolved in water.
When they are converted to silk the structure changes and becomes immensely stable. This transformation occurs in part with a decrease in pH values along the duct.
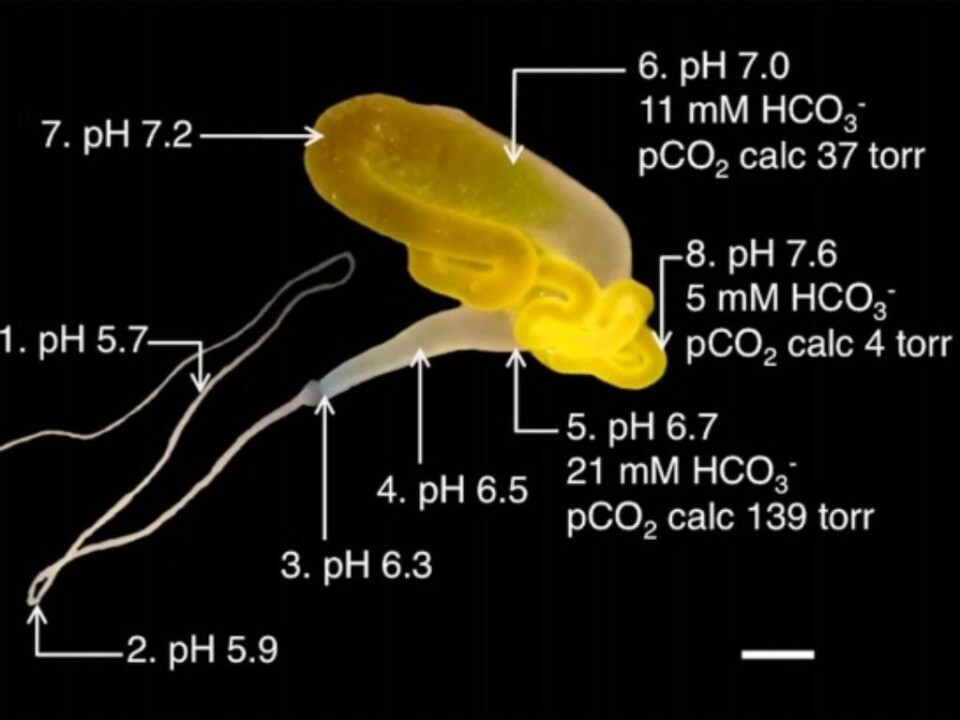
The current breakthrough was made by the team of scientists through their use of ion selective microelectrodes to gauge the gradient of pH levels in several spots along the duct.
They found that the pH level sank gradually from a quite neutral value of 7.6 to a much more acidic 5.7 halfway through the duct.
Significantly, the researchers discovered that this drop in pH was retained by the enzyme carbonic anhydrase. This enzyme converts carbon dioxide and water to hydrogen carbonate and protons, which make the immediate environment more acidic. The process can also be reversed.
They also found that the carbon dioxide pressure mounted on the way down the duct and this can also have an effect on the spidroin.
This process transforms the spidroin protein. The neutral pH value kept the proteins from binding and clustering.
When they are pressed down into the acidic environment in the duct, the proteins begin to bind and “unfold” to create silk fibre.
“The spider has evolved unique mechanisms for controlling the water solubility of the proteins and their ‘clustering’,” write Rising and Johansson.
Industrial spider silk?
Spider silk is a strong and useful material which would be a fabulous component of all sorts of manufactured products, for instance extremely strong cables or bullet-proof vests.
“Because of costs, we think artificial spider silk will first be used in regenerative medicine, where we try to repair and restore body tissue,” write Rising and Johannson.
No other synthetic materials can currently match the properties of spider silk. It will also be a long time before it can be produced on a large scale. Spider farms are certainly not the answer.
“Spiders are territorial and eat one another if they placed together.”
Anna Rising and Jan Johannson write that we can create small amounts of silk proteins with the help of bacteria but no one has succeeded at joining these together to a thread that comes anywhere near matching the fabulous properties of the real thing made by spiders.
“Our findings can be used in imitating the spider’s method of producing silk and in a more natural way than has been previously possible,” say Rising and Johannson.
The researchers add that first we would have to produce the silk proteins in a more cost-effective, large scale way, and then design a good spinning machine.
------------
Read the Norwegian version of this article at forskning.no
Translated by: Glenn Ostling







