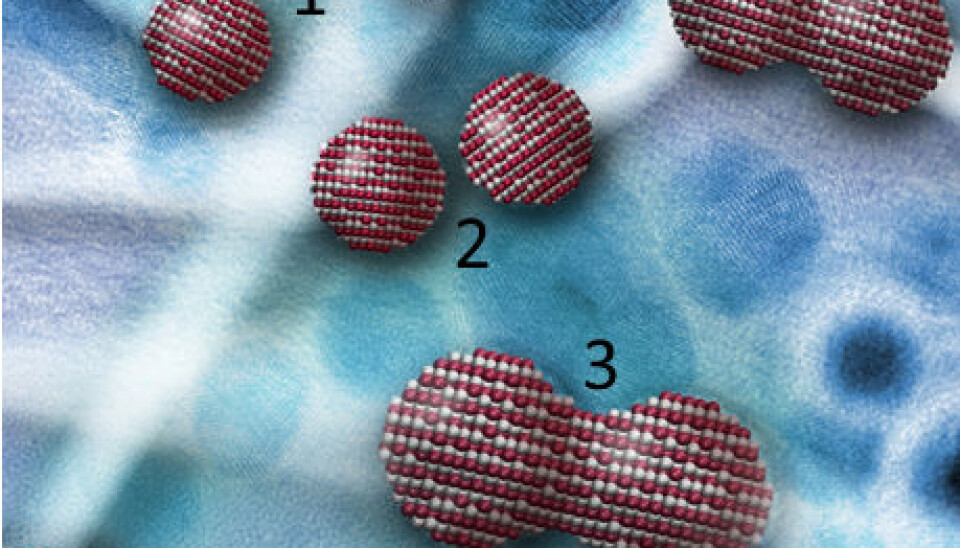
Crystals connect like LEGO bricks
Crystals grow as a result of their nanoparticles locating each other and connecting at the exact spot where the atoms fit together.
Nanoparticles are tiny crystals. In liquid they move around more or less randomly. Studies of dry crystals, on the other hand, have indicated that while in liquid the crystal grew by means of the nanoparticles finding a spot where their crystal lattices match perfectly and making a so-called oriented attachment.
This is similar to a LEGO brick that fits perfectly on top of another.
One could imagine the nanoparticles as microscopic LEGO bricks, where the pins are positively charged atoms and the holes are negatively charged atoms.
At a certain point they are in perfect alignment, and the strong attraction between the two opposing charges makes the bricks connect to form a larger crystal.
Self-assembling LEGO bricks

Up to now it has not been possible to observe this process by studying dry crystals. But now, using a fluid cell inserted into a powerful electron microscope, an American research team with Danish participation has observed how iron oxyhydroxide nanoparticles connect and form larger crystals.
“When one particle meets another, it checks whether it fits. If it doesn’t, it will keep moving around until it finds a match,” explains Associate Professor Cathrine Frandsen, of the Department of Physics at the Technical University of Denmark (DTU).
“It’s as if the nanoparticle feels that the atomic charges need to match. It has very few ways of doing that. It’s a bit like LEGO bricks; there are only a few ways to make them fit perfectly.”
An important difference, however, is that the nanoparticles are actually self-assembling LEGO bricks.
An understanding of crystal growth can be valuable
Using nanoparticles as building blocks, we may be able to create materials with entirely new forms and characteristics.
Frandsen, who has spent a year working with the research team from the University of California, Berkeley, researches into nanoparticle properties with an eye to designing new materials.
"If you understand how crystals grow by means of nanoparticles attaching themselves to each other, you can use this knowledge to design new materials in which the nanoparticles are the building blocks," she explains.
“It’s amazing that we can now see directly how the particles attach to each other.”
So far it has not been possible to see how the particles moved and oriented themselves. But now the research team is able to describe this process.
This is important to their understanding of crystal growth as it occurs both in nature and in the laboratory, as well as to their ability to design new materials.
”Using nanoparticles as building blocks, we may be able to create materials with entirely new forms and characteristics,” says Frandsen.
“For example, very thin, elongated and branched structures, which can be used in future electronic devices, perhaps as small powerful magnets in mobile phones or as filters to purify water for toxic ions.”
The findings from the study have just been published in the journal Science.
--------------------------------
Read this article in Danish at videnskab.dk
Translated by: Dann Vinther






